Development of the Atom
By: Cindy Nguyen
Early Greeks
332 BCE - 450 BC
Democritius
400-450 BC
Came up with the theory that
all materials
are made up of
indivisibly small "atoms"
leads to the "atomos" model
Aristotle
332 BCE
Believed that all matter was made up
of the 5 basic elements:
earth, fire, air, water and ether
Didn't believe in the theory
that all matter is made of atoms
1800's
John Dalton
1808
Stated 3 things:
1. all matter was made of small,
indivisible particles called "atoms
2. the atoms in a given element all have
unique characteristics and weight
3. there are 3 different types of atoms that
exist: simple (elements), compound (simple
molecules), and complex (complex molecules)
Came up with the "Marble Model"
--> Also known as the "Billiard Ball
Model"
Dalton thought of the atom
to be a ball-like structure
Michael Faraday
1839
Discovered that atoms had an electrical
component to them
Julius Plucker
1859
A pioneer in the investigations of
cathode rays that eventually led
to the discovery of the electron
A cathode ray is a stream of
electrons that are seen in
vacuum tubes
Dmitri Mendeleev
1869
Created the periodic table
The elements where arranged in order
of increasing atomic weight
George Johnson Stoney
1879
Determined the charge of the atom
--> also known as the "particle of
electricity"
Introduced the term "electron"
Eugen Goldstein
1886
Discovered positive particles by using a tube
filled with hydrogen gas
The positive particle that he discovered
is now known as "proton"
Henri Becqurel
1896
Discovered radioactivity by accident
Radioactivity: The release of energy
from the decay of the nuclei of
certain types of atoms and isotopes
J.J Thomson
1897
Created the "plum pludding model"
The model contains:
- negatively-charged electrons
- a positively charged "soup"
The plums = electrons
The pudding = sphere of the model
Discovered the electron
Marie and Pierre Curie
1898
Discovered Polonium and Radium
"Polonium" is named after Poland, which was
Marie's homeland
Marie Curie concluded that
radioactivity originates
within the atom itself
(something was happening inside
the atom itself)
Radioactivity isn't dependent on the way that
the atoms are arranged to form molecules
1700's
Isaac Newton
1704
Suggested that atoms are held together
with attractions
These attractions are known as "forces"

1900-1920's
Max Planck
1900
presented a theory known as
"Planck's Quantum Theory"

Hypothesized that matter emits/absorbs
energy in small packets of energy called
"quantum"
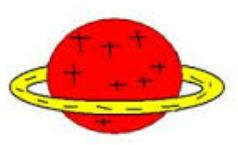
Nagaoka Hantaro
1904
Came up with the Saturnian model
Contains:
- small nucleus (orbited by electrons)
It was also the first ever model to include
the nucleus core
Robert Millikan
1910
Discovered the charge of an individual electron
Also discovered the fact that all electrons carry the
same amount of negative charge
Ernest Rutherford
1911
Discovered atoms contained a positively charged
nucleus that is much smaller than the actual atom
Also discovered about the existence
of gamma, alpha, and beta rays
His model is known as the nuclear model
Contains:
- protons and neutrons (makes up
most of the mass in the atom)
Conducted a goid foil experiment where a piece of
gold foil was hit with alpha particles
--> alpha particles have a positive charge
Rutherford did not expect the particle to go through gold,
however they did.
--> He concluded that the gold atoms were
mostly empty space
This means that there is large empty space around the nucleus, meaning that the nucleus is very small!
Neils Bohr
1913
Modified Rutherford's model to create his own
representation of the model
Modified Rutherford's model to create his own
representation of the model
Discovered that the electrons don't fall into the nucleus
Added a condition stating that the shells have
fixed energy. Due to this condition, he solved the
stability problem of the electrons
His theory was that the electrons orbit the nucleus
at fixed energy levels
Henry Moseley
1913
Came up with "Moseley's Law" where the law
gave scientists a more accurate way to
organize elements
Discovered an accurate mathematical connection
between the frequency of x-ray radiation
and an element's atomic number
Frederick Soddy
1913
Announced the concept that atoms can
have be the same chemically, but have
different atomic weights
These related atoms are known as
"isotopes"
Francis William Aston
1919
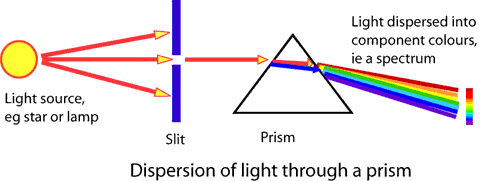
Developed the mass spectrograph to map
the different isotopes
Discovered the isotopes of the light elements
using his spectrograph
A spectrograph is a device that separates a signal into its component wavelengths (think of a prism splitting white light into colours)
1921-1929
James Franck
1920-1933
The Franck-Hertz experiment
gave proof for Bohr's theory that
an atom can absorb internal
energy (quanta) in only
precise and definite amounts
The experiment consists of a vacuum
tube designed to study the energetic
electrons that flew through a
thin vapor of mercury atoms
The experiment demonstrated the
existence of excited states in
the mercury atoms
Arthur Compton
Founder of the Compton Effect
Demonstrates the particle nature
of electromagnetic radiation
Electromagnetic radiation is made when
an atom absorbs energy which allows
for one or more electrons
to change their place within the atom
Louis De Broglie
1923-1924
Introduced the idea that particles
(such as electrons) could be described as
waves
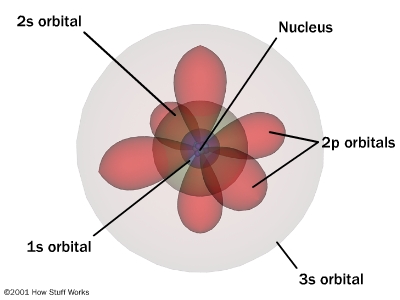
Proposed the wave-mechanical model (1920s)
with Erwin Schrodinger to determine
that Bohr's model wasn't useful to determine
the locations of the electron
Model proposes that each electron circling an atom's
nucleus occupies a specific orbital (s,p,d,f)
and spins in a certain direction
Werner Heisenberg
1925
Contributed to atomic theory by
formulating quantum mechanics
in terms of matrices (sets of numbers)
Founded the uncertainty principle
States that we can't know both the position
and speed of a particle
Max Born (1925)
After Heisenberg's work, he contributed to
the further development of
quantum mechanics
Proved that Schrodinger's wave equation
could be interpreted by giving statistical
predictions of variables rather than
exact numbers
Erwin Schrodinger
1926
Formulated a wave equation that accurately calculated
the energy levels of the electrons in the atom

Proposed the wave-mechanical model
with Louis de Broglie in the 1920s
Paul Dirac
1928
Developed a relativistic quantum theory that
produced results that he believed
were produced by an electron-like particle
with a positive charge
Equation described how particles move slower
than the speed of light
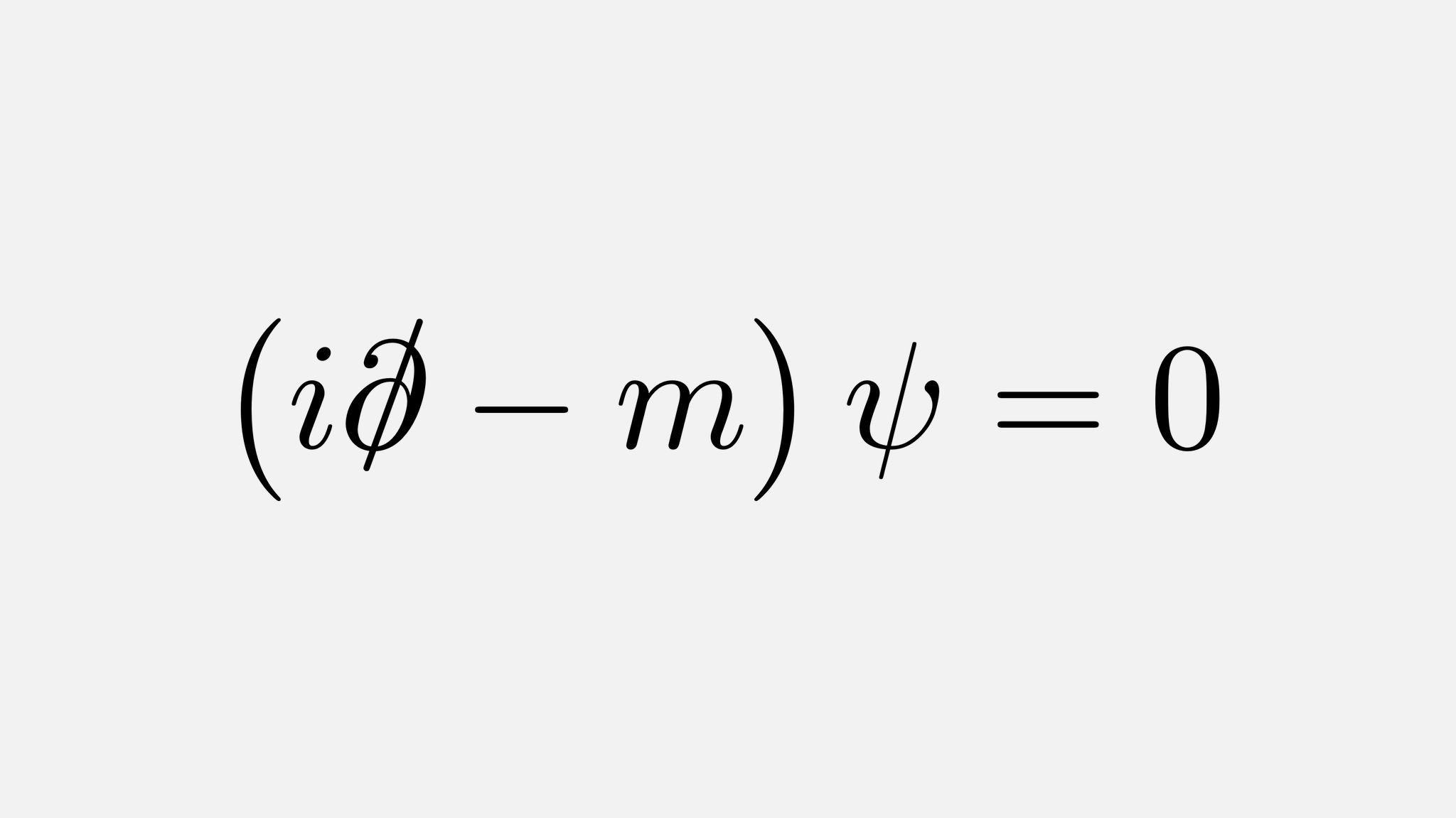
Egil Hylleraas
1929
Applied Schrodinger's equation to
the Helium atom (that has 2 electrons)
--> Bohr's model couldn't explain for atoms
that had more than 1 electron
Hylleraas was also able to explain the
electron's energy
1930-1949
James Chadwick
1932
Known for his discovery of the neutron
(proved their existence)
Enrico Fermi
1942
Showed that almost every chemical element
could be made radioactive
His work on radioactivity soon led to the
discovery of nuclear fission
During nuclear fission, an atom's nucleus is
split apart
--> the split causes energy to be released
Maria Goeppert-Mayer
1949
Developed the nuclear shell model where
nucleons (neutrons + protons) were
distributed in shells with diff energy levels
Developed the model with her friend,
Hans Jensen

Hans Jensen
Model explains the distribution of energy
levels into various atom shells
Displays observations of the direction where
the nucleons (protons + neutrons) rotate their
own axes and around the nucleus' center
The atomic nucleus is thought to have
a structure of shells/spherical layers
that contains those nucleons