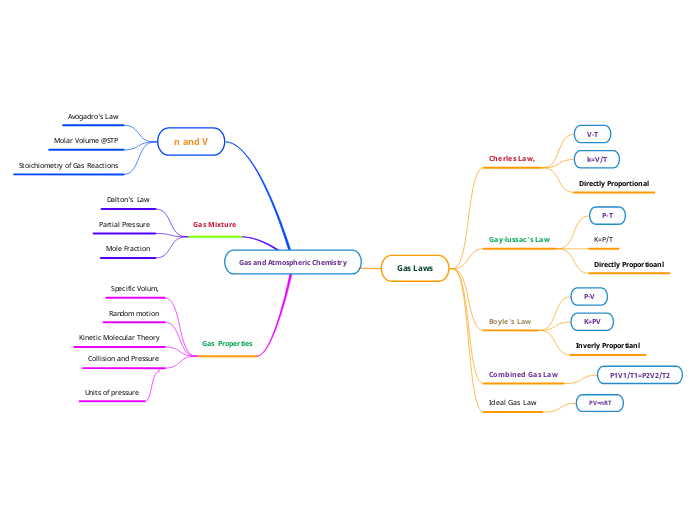
Gas and Atmospheric Chemistry
Gas Laws
Cherles Law,
V-T
k=V/T
Directly Proportional
Gay-lussac's Law
P-T
K=P/T
Directly Proportioanl
Boyle's Law
P-V
K=PV
Inverly Proportianl
Combined Gas Law
P1V1/T1=P2V2/T2
Ideal Gas Law
PV=nRT
n and V
Avogadro's Law
Molar Volume @STP
Stoichiometry of Gas Reactions
Gas Mixture
Dalton's Law
Partial Pressure
Mole Fraction
Gas Properties
Specific Volum,
Random motion
Kinetic Molecular Theory
Collision and Pressure
Units of pressure