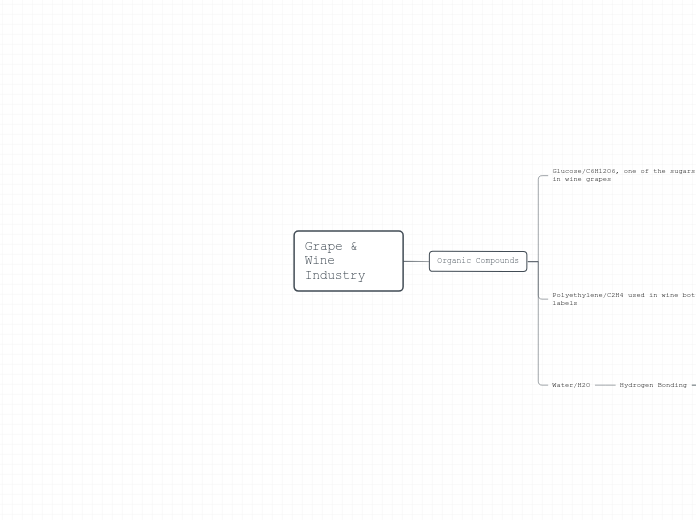
Grape & Wine Industry
Organic Compounds
Glucose/C6H12O6, one of the sugars found in wine grapes
Polar covalent bond
Soluble w/ other polar molecules, therefore soluble in water
Strong intermolecular forces because of dipole force therefore solid at room temp.
Stronger intermolecular forces between the solute and solvent molecule, the greater the solubility
Stronger intermolecular forces therefore higher boiling and melting point
Bonds with polar end of emulsifiers
Polyethylene/C2H4 used in wine bottle labels
Non-polar covalent bond
Soluble w/ other nonpolar molecules, therefore not soluble in water
Weak intermolecular forces because nonpolar molecules only have London forces (weakest intermolecular force) therefore liquid at room temp.
Weaker intermolecular forces therefore lower boiling and melting point
Bonds with non-polar end of emulsifiers
Water/H2O
Hydrogen Bonding
Only in a bond between hydrogen, and fluorine, oxygen or nitrogen. Creating an extremely strong dipole because of extremely high electronegativity.
Strongest intermolecular force