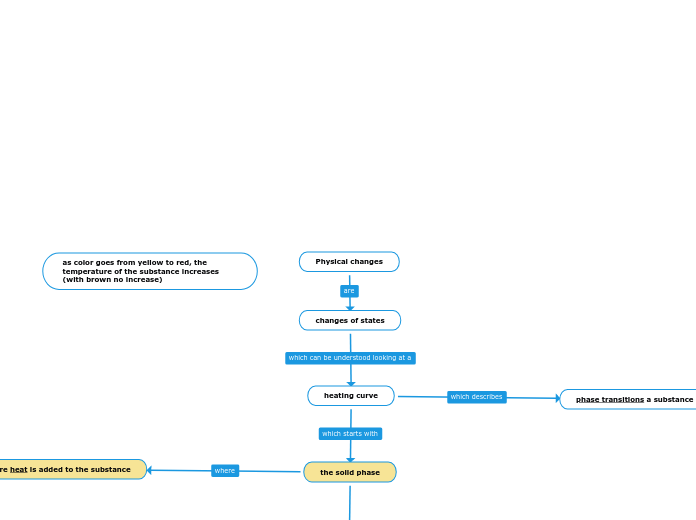
Physical changes
changes of states
heating curve
the solid phase
the melting phase
the liquid phase
the boiling phase
the gas phase
as more heat is added to the substance
as more heat is added to the substance
as more heat is added to the substance
as more heat is added to the substance
as more heat is added to the substance
phase transitions a substance undergoes
heat (thermal energy in motion) is added
particles move very quickly
many collisions with each other
only a small amount of heat
to raise 1g of the substance by 1 degree Celsius
specific heat capacity
an increase in the average kinetic energy (temperature)
the particles are vibrating faster
starting to weaken its intermolecular forces
particles are closely packed together
a small amount of heat
raise 1g of the substance by 1 degree Celsius
specific heat capacity
no increase in the average kinetic energy (temperature)
added energy is stored as potential energy
molar heat of fusion/vaporization equations can only be used
amount of heat required to undergo certain changes
an increase in the average kinetic energy
vibrate AND rotate
totally overcome its intermolecular forces of attraction
start escaping into the gas phase
moderately spaced out and have a moderate speed
larger amounts of heat
increase 1g of the substance by 1 degree celsius