suspension
good suspension
particle size
uniform=narrow particle size distribution
particle with the samilar density
as medium can afford having
more particle because the
sedimentation rate is slower
stable
differentiating factor in suspension
slow sedimentation rate
slow to settle

Sedimentation rate
particle size
too small
slow to settle
will form cake
too large
fast to settle
easy to re-disperse
10 x increase in particle size
results in 100x increase in
sedimentation rate
WANT the particle size to
be as narrow as possible
particle density
Density of particle less
than medium (water)
float
Density of particle a
>> than medium
fast to settle
WANT particle density to be a
little but lower than medium
slow sedimentation rate for stability**
easy to re-disperse
the stokes equation cannot determine
if particle will readily re-disperse
easy consistent pour
vehicle viscosity
adjusted in accordance
to particle size and density
to minimize sedimentation
rate
adjusted via addition
suspending agent
thickening agent
thixotropy is a
good vehicle
high stress --> less viscous
easy to disperse
over time viscosity increases
slow to settle
vehicle
not heavily adjusted to
achieve good suspension
thixotropy is a good vehicle
under high stress become less viscous
easy to disperse
overtime viscosity increases
slow to settle
flocculation
loose structure when settle
FLOC
thus easily redistributed
common use
Antiacid - are inorganic particle that are insoluble in water
antiimicrobial- unstable in solutions
Why
when. liquid is required but
drug is not dissolve in liquid or
unstable in solution
suspension can improve the stability
of undissolved drug
dose flexibility
mask the tase
better dissolution and absorption than tabelts
slower dissolution and absorption than tablets
definition
suspension
finely divided particles distributed somewhat uniformly throughout a vehicle in which the drug exhibits minimum solubility
Particles( clubs) surrounded by water molecule
drug molecule surrounded by drug molecule
will not dissolve
WILL eventually settle over time
cant see through
vehicle
dispensing medium
external liquid phase
drug
the dispered phase
internal phase
suspensiod
classification
3 main type
oral
oral suspensionn better flexibility in
dosing than oral tablet
most common
externally applied
lotion
parenteral
most common insulin zinc suspension
liposomal doxorubicin
suspension limitation in the blood
is the particle size of the drug.
particle size can be determined by
light transmission through the suspension
small particle size = light passes through
large particle size = light does not passes through
must be low particle size == looks like solution
fastes growing
particle size
Colloidal
< 1micron
nanometer range
suspension/solution
typically do not settle under gravity b/c so
small that particle remain suspended
may look like solution
coarse
over 1 micron
settle under gravity
always a suspension
preparing
Wetting to create barrier
surfactants
cover particles and form a bridge
between the lipophilic particle and
the hydrophilic medium
amphiphilic
used to reduce interfacial tension
creating a barrier to avoid "caking"
prevent particles from
sticking to each other
too much
bad tase
dissolution of drug
Extemporaneous
compound
from solid--> suspension
less stable
process
dispersed particle are wetted---> paste
add remaining vehicles in parts while mixing
settling
all suspension particle
WILL eventually settle
once particles settle can form a
hard cake that does not re-distribute
easily and is to be avoided
avoid formation of "cake"
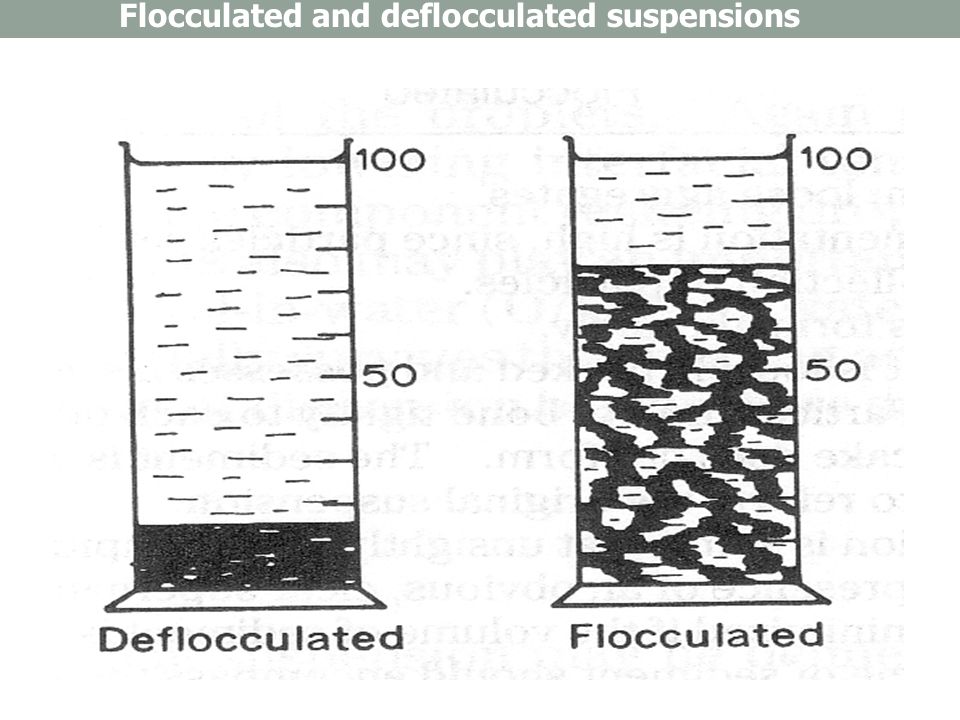
flocculation
made by additive
clays
interfere with other particle creating barrier
thus help support the floc structure once it forms
most common
cons: add volume
Suractants
prevents particle from sticking
done by wetting
adjusting pH and electrolytes
promote int interactions for particle
to repple and prevent from clumping forming cake
most sophisticated
promote loose settling
we cannot prevent settling
might as well control it
artificially form when
particle settle to re disperse
process to form "floc", a loose structure
due to particle settling very fast
weak particle particle forces
fast settling but "fluffy" ( loose structure) = Floc
easily redistributed
disadvantage
lack drug stability data
cause clumps floating around
I-clicker
A flocculated system settles very rapidly not allowing enough time for accurate dosing. what would you try first to fix
A)reduce particle size of disperse phase
we already have a flocculated system! change is particle size can affect the flocculated systemm
B) increase viscosity
flocculated solution is a very loose suspension thus increasing viscosity will allow the drug to settle in a slower rate. giving more time for accurate dosing
C) use homogenizer to better breakdown the drug particle
RUIN the flocculated suspension??
d) add a wetting agent
wetting agent is used to make a flocculated system
