realizată de Liang Koo Vilca 4 ani în urmă
275
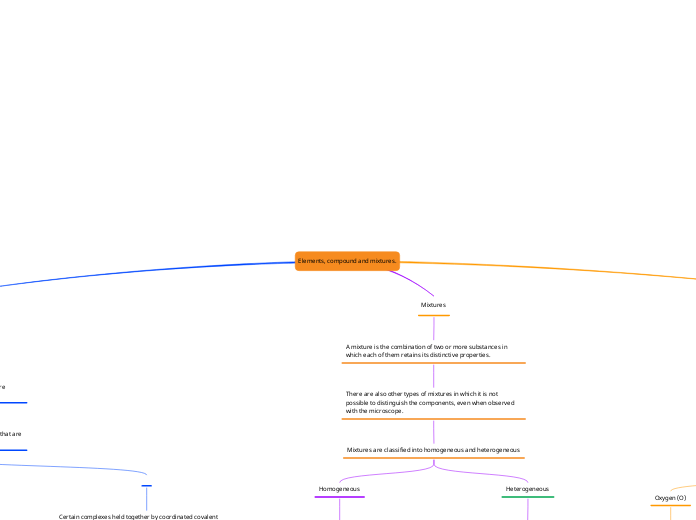
Elements, compound and mixtures.
realizată de Liang Koo Vilca 4 ani în urmă
275

Mai multe ca acesta
Examples of elements:
Silver (Ag)
Nitrogen (N)
Carbon (C)
Phosphorus (P)
Oxygen (O)
Mixtures are classified into homogeneous and heterogeneous
Heterogeneous
A heterogeneous mixture is a combination of 2 or more elements or substances that can occur in any state of matter, in which its components can be identified.
Homogeneous
A homogeneous mixture is the combination of 2 or more elements or substances. Homogeneous mixtures are characterized by being uniform, that is, the elements that compose them are not distinguishable with the naked eye.
A chemical compound is made up of molecules or ions that are stable together.
Certain complexes held together by coordinated covalent bonds.
Links between silver (Ag) atoms. Bonds between gold (Au) atoms. Bonds between cadmium (Cd) atoms.
Intermetallic compounds joined by metallic bonds.
Links between silver (Ag) atoms. Bonds between gold (Au) atoms. Bonds between cadmium (Cd) atoms
Ionic compounds linked by ionic bonds.
Magnesium oxide (MgO)
Molecules joined by covalent bonds.
Pure hydrogen (H2). H-H (a single link)
examples: Pure hydrogen (H2). H-H (a single link)