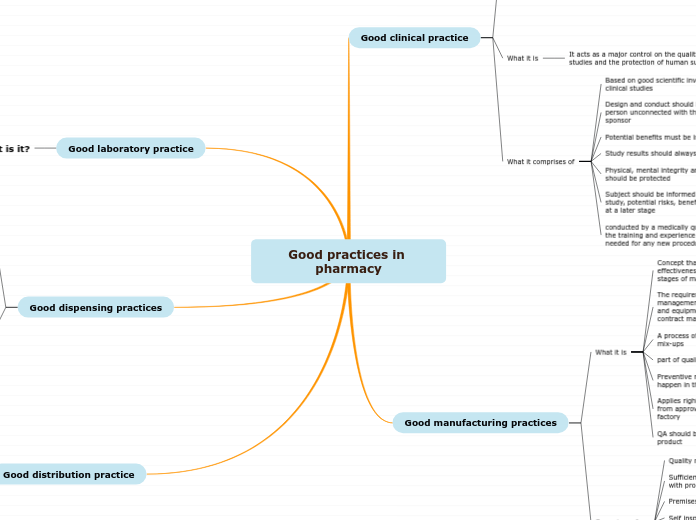
Good practices in pharmacy
Good clinical practice
For clinical trials
Clinical trials in Singapore
All clinical trials on medicinal products conducted in Singapore requires a clinical Trail Certificate
Additional info
The HSA through its Health Products Regulation Group, provides advice on the licensing of clinical drug trials
New applications for clinical trials certificates
Amendments to clinical trials protocols and informed consent documents
Serious adverse event report
Requests for CTC extensions
Because of
Increase in clinical research activities in Singapore
A need to grant appropriate protection to ensure the safety and well-being of trial subjects
Purpose
Set ethical and scientific standards for the conduct of clinical trials
Serves as an assurance that results obtained from clinical trials are credible
IRB
A committe that ensures health and safety of patients
What it is
It acts as a major control on the quality of clinical studies and the protection of human subjects
What it comprises of
Based on good scientific investigation including pre-clinical studies
Drug should be tested rigorously in labs in cell cultures and animals and healthy volunteer first
Design and conduct should be scrutinized by a person unconnected with the investigator and sponsor
Potential benefits must be in proportion with risks
Study results should always be reported accurately
Physical, mental integrity and privacy of the subject should be protected
Subject should be informed of the details of the study, potential risks, benefits and rights to withdraw at a later stage
conducted by a medically qualified person who have the training and experience in clinical trials and skill needed for any new procedure being investigated
Good manufacturing practices
What it is
Concept that covers the assurance of the safety and effectiveness of pharmaceuticals during all the stages of manufacturing
The requirements to cover such topics as quality management, personnel, documentation, premises and equipment, materials handling, validation, contract manufacturing, and self inspection
A process of preventing cross-contamination and mix-ups
part of quality assurance
Preventive measures tat is designed to ensure things happen in the correct manner
Applies rights from purchase of starting materials from approved suppliers until finished products leave factory
QA should be appropriate to the intended use of product
Comprises of
Quality management/ Quality System
Sufficient no. of qualified, experienced personnel, with proper training and hygiene
Premises and equipment
Self inspection audit
Documentation of specifications for raw materials/ packaging materials/finished products
Product process- proper handling of materials throughout process
Product complaints
Good laboratory practice
what is it?
Adequate facilities
Apparatus/ reagents must have validation, maintenance and proper storage
main area of activity must be covered by SOP
Physiological and biologicla testing system
Qualified Personnel with training
Proper receipt, handling, sampling and storage of all test and reference materials
proper storage and retention of records that allows for traceability
Good dispensing practices
What it is
Ensure quality of dispensed or compounded product by covering areas such s cleanliness, appearance, documentation, equipment, manipulative techniques, ingredients, calculations, counting etc
What it comprises
Cleanliness of drug, premises/bench/equipment/store
Documenting procedures and result
storage
Appearance: lab coat, hair, professional
equipment for extemporaneous preperations
counting devices must be kept clean daily and electronic counting equipment must be maintained for accuracy for accuracy
Good distribution practice
What it is
A concept that covers the assurance of the safety and efficacy of pharmaceuticals from the time they leave the factory gates to the point at which they are used by the patients
What is comprises of
Authorized/quality products
safe and secure storage conditions in warehouse as well as during transport
Avoid cross contamination
Right Product, Right Place, Right Time
FIFO system in stock movement
Full traceability in the distribution chain
Effective recall system