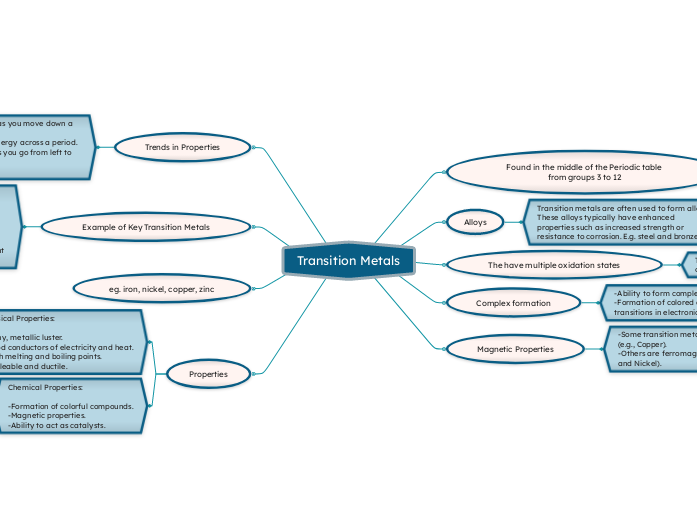
Transition Metals
Found in the middle of the Periodic table from groups 3 to 12
Alloys
Transition metals are often used to form alloys. These alloys typically have enhanced properties such as increased strength or resistance to corrosion. E.g. steel and bronze.
The have multiple oxidation states
Transition metals can form multiple positive oxidation states.
Complex formation
-Ability to form complex ions -Formation of colored compounds due to d-d transitions in electronic levels.
Magnetic Properties
-Some transition metals are paramagnetic (e.g., Copper).
-Others are ferromagnetic (e.g., Iron, Cobalt, and Nickel).
Trends in Properties
-Increase in Atomic Size as you move down a group.
-Increase in Ionization Energy across a period.
-Decreasing Reactivity as you go from left to right
Example of Key Transition Metals
Iron (Fe): Found in hemoglobin, important in construction.
Copper (Cu): Excellent conductor, used in electrical wiring.
Titanium (Ti): Known for strength-to-weight ratio, used in aerospace.
Zinc (Zn): Used for galvanizing steel to prevent rusting.
eg. iron, nickel, copper, zinc
Properties
Physical Properties:
-Shiny, metallic luster.
-Good conductors of electricity and heat.
-High melting and boiling points.
-Malleable and ductile.
Chemical Properties:
-Formation of colorful compounds.
-Magnetic properties.
-Ability to act as catalysts.