по seyed Sajjadi 3 лет назад
238
Gas and Atmospheric Chemistry
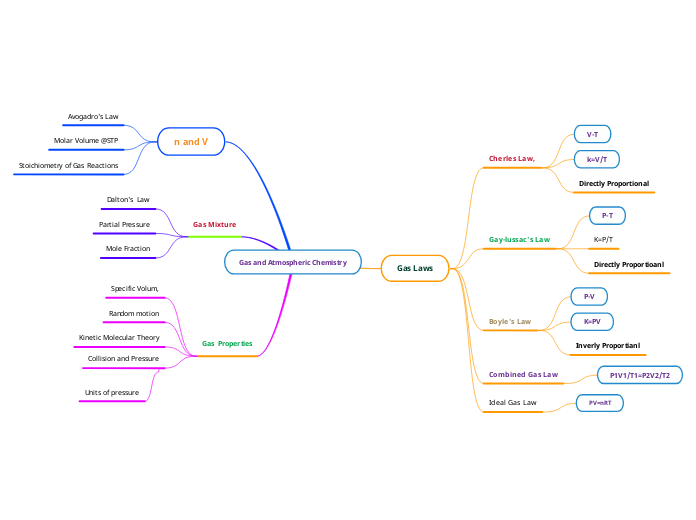
Understanding gas behavior involves various fundamental principles and laws. Key concepts include the Ideal Gas Law, which relates pressure, volume, temperature, and the number of moles of gas using the equation PV=nRT.