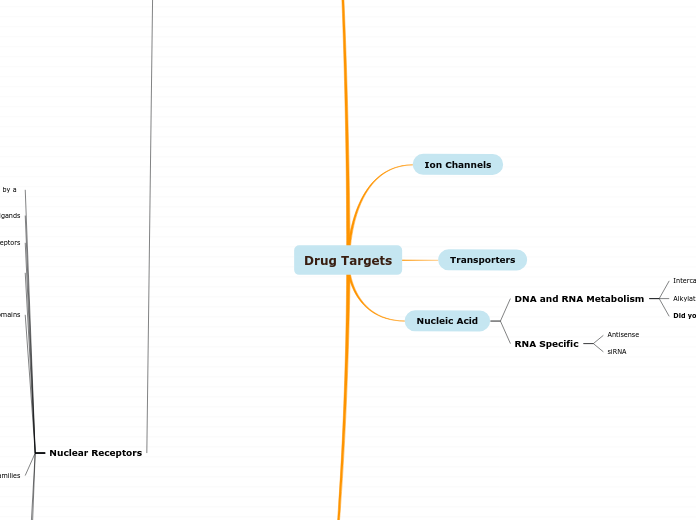
Drug Targets
Ion Channels
Transporters
Nucleic Acid
DNA and RNA Metabolism
Intercalating agens
Alkylating agents
Cross-link strands
Did you know?
RNA Specific
Antisense
siRNA
Classical Receptors
Ligand Gated Ion Channel
Binding of the endogenous ligand or agonist drug changes conformation, straightening kink in alpha helixes, allowing passage
Cation Selective
Aqueous pore
Serotonin/5-hydroxytryptamine type 3 (5-HT3)
Anion selective
Tend to be not selective for anions, but mostly mediate chord transport
Gamma aminobutyric acid receptor (GABA type A)
Receptor activation
Neurotransmitters like Act/Glutamate
Very fast response time
No-mediator activation
Conductance doesn't vary between endogenous or agonist drugs
Period of opening varies
Possible desensitization
When channel closes but ligand remains bound
Extra receptors
Arachadonic acid sensitive receptors
ATP and calcium binding receptors
membrane localized, ligand binding domain directly coupled to larger channel complex in an oligomeric assembly of subunits surrounding central pore
G-Protein-Coupled Receptors
Single PP of ≤1100 residues which form seven transmembrane α-helices amongst other structures
Other residues form an extracellular domain at the N-terminal and a cytoplasmic domain at the C-terminal
g-protein coupled receptor agonists
Rapid effect (neurotransmitters)
Sub families
Rhodopsin
Largest group
Short extracellular (EC) tail (N-terminal)
Ligans bind to helices or extracellular hoops
Examples
Amine neurotransmitters, neuropeptides, purines, protanoids, cannabinoids
Secretin/Glucagon
Receptors for peptide hormones (calcitonin)
Intermediate EC tail with ligand binding domain
Metabotropic glutamate/Ca sensor
Smallest group (GABA B receptors)
Examples
ACh
5-HT
Dopamine
Opioids
Olfaction
G-protein activation
Act as messengers for the receptors coupled to them.
Alpha, beta and gamma subunits anchored to the membrane by lipid residues
Freely diffuse around the cytoplasmic surface of the cell membrane and associate with ligand bound receptors.
Conformational change to expose high affinity binding sites for the G-protein trimer complex
GDP occupies a site on the alpha subunit.
GDP is converted to GTP
Signals the release of the activated GTP-bound alpha subunit from both the receptor and the beta and gamma subunits the latter two remaining bound together
alpha subunit is then free to bind a target effector protein.
alpha subunit has intrinsic GTP-ase activity and eventually self hydrolyses GTP to GDP thus inactivating itself
4 Families
G alpha s
Stimulating
G alpha i
Inhibitory
G alpha 12
G alpha q
G-protein modulation of effector molecules
One effector controlled by different G-protein complexes
Desentitization
Receptor phosphorylation
Down regulation
Endocytosis (receptor internilization)
C-terminal cytoplasmic tail, serine/theonine rich and prone to kinase activity
PKA, PKC
GPC receptor kinases
membrane localized , work via activation of g-protein which translocate to and activate effector ion channel or enzymes, mono or oligomeric, 7 transmembrane helices with intracellular g-protein coupling domain
Drug shares endogenous ligand binding site
Tyrosine kinase-linked receptors
Large extracellular ligand-binding domain linked to an intracellular domain by a single membrane spanning helix.
Control inflammation, tissue repair, cell cycle progression, apoptosis and immune response.
initiated by ligand binding followed by dimerization of the receptor.
Autophosphorylation of tyrosine residues on the cytoplasmic portion of the receptor
phosphorylated Tyrosine residues bind and activate a variety of intracellular signaling proteins via interaction with regions called SH2 domains
Phospholipases and other protein kinases
Receptors
Receptor Tyrosine Kinases
Growth factors
Toll-like receptors
Ligans bind to helices or extracellular hoops
Examples
Amine neurotransmitters, neuropeptides, purines, protanoids, cannabinoids
Serine/threonine kinases
Transforming growth factor
Cytokine receptors
No integral kinase moieties
Associate with cytosolic tyrosine kinases
Examples
growth factors
cytokines
insulin
bacterial lipopolysaccharides
Kinase-linked receptor transduction mechanisms
Associate with independent cytoplasmic kinase proteins
Dimerization for both receptor types on ligand binding.
conformational change
Then phosphorylation
Tyrosine Kinase-linked receptors
Receptor’s intrinsic kinase activity phosphorylates proteins bound to the activated intracellular domains of the receptor.
SH2 domain
Cytokine receptors
activation of the receptor by cytokine binding to the extracellular portion
conformational change in the intracellular portion that allows binding of an independent cytoplasmic kinase
Jak Stat signalling pathway is common to many cytokines.
membrane localized, having intrinsic protein kinase activity or directly linking to free protein kinases, a single transmembrane helix linking extracellular domain to intracellular kinase domain
Nuclear Receptors
Monomeric with a DNA recognition and binding domain and a ligand binding domain connected by a flexible linking hinge.
Ligands
Many hormones and vitamins, such as vitamin D.
orphan receptors
No defined ligand
RXR receptor (similar to vitamin A receptor)
Directly interact with DNA and modulate transcription
Domains
C-terminal domain bears a region that governs the nuclear localization of the receptor.
N-terminal region controls interaction of the receptor with co-activator or co-repressor proteins that modulate the transcriptional functions of the nuclear receptor
Sub families
Class I
Cytoplasm
Forming homodimers on ligand binding before translocating to the nucleus
receptors for steroid hormones
other endocrine mediators such as oestrogen, progesterone and androgen.
Class II
Nucleus
form heterodimers with RXR, the retinoid x receptor.
fatty acid and cholesterol sensitive receptors
xenobiotic receptors
causes the induction of drug metabolizing enzymes such as cytochrome P3A
Hybrid Class
members of the Class II receptors form obligate heterodimers with RXR
thyroid hormone receptor and the vitamin D receptor.
Mechanism
Class I
Ligand binding to cytoplasmic hormone receptors initiates dissociation from the heat shock protein
allows binding to specific DNA sequences called hormone response elements on the target genes
receptor dimers recruit ancillary proteins, co-activators and co-repressors that either promote or suppress gene transcription.
Class II
initiates heterodimerization generally with the retinoid X receptor as a partner.
Co-activator proteins and co-repressor proteins also regulate
often specifically causes dissociation of a co-repressor molecule
allowing the binding of a co-activating protein.
ntracellular, translocate to nucleus, act via direct interaction with DNA, monomeric structure with separate receptor and DNA binding domains