磷 фосфор
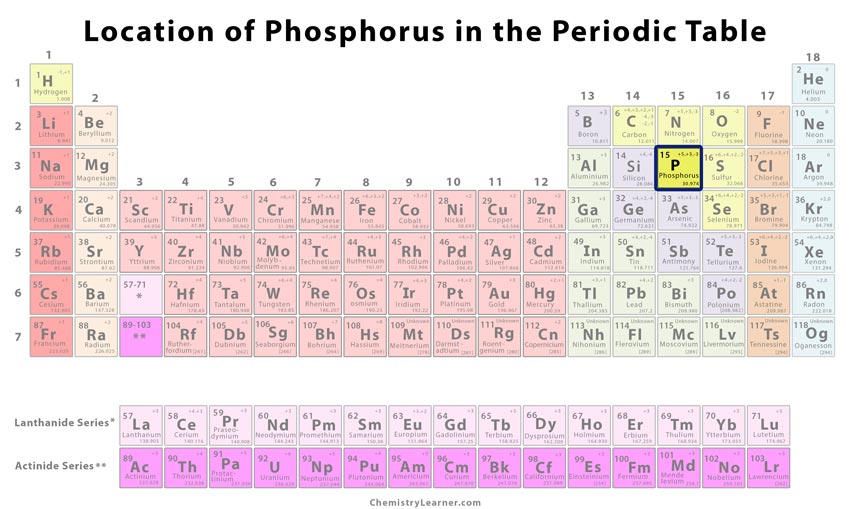
Location on the Periodic Table

Historical information

Phosphorus (name taken from Greek mythology, Φωσφόρος meaning 'light-bearer) was discovered in 1669 by a German alchemist Hennig Brand. He was searching for gold and processing urine in search of a compound that would turn ordinary metals into gold. This is how he accidentally discovered the 15th element!

Facts About Phosphorus
Isotopes = 22 (1 stable)
Phosphorus is a nonmetallic chemical element of the nitrogen family that at room temperature is a colourless, semitransparent, soft, waxy solid that glows in the dark.
Phosphorus is usually produced from phosphate rock that is mined and processed into various products. There are different methods for phosphorus extraction, such as wet process, extraction, precipitation, and recovery from waste.
Impacts of Phosphorus Use
It contributes to major water quality issues, such as the formation of oxygen-free dead zones

Idea
https://www.livescience.com/37057-global-warming-effects.html https://www.chemistrylearner.com/phosphorus.html#google_vignette https://www.britannica.com/science/phosphorus-chemical-element https://en.wikipedia.org/wiki/Phosphorus Phosphorus Facts - Element Characteristics & Properties (thoughtco.com) https://www.britannica.com/science/phosphorus-chemical-element https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_Chemistry_(Zumdahl_and_Decoste)/18%3A_The_Representative_Elements/18.09%3A_The_Chemistry_of_Phosphorus https://link.springer.com/chapter/10.1007/978-981-10-8031-9_5 https://phys.org/news/2023-07-phosphorus-sustainable-stakeholders.html https://www.famousscientists.org/glenn-seaborg https://en.wikipedia.org/wiki/Chemical_element
Countries Phosphorus is Most Abundant

Morocco

China
.jpg)
South Africa
Chemical and Physical Properties of Phosphorus
PHYSICAL: The most common forms are white phosphorus and red phosphorus but there is also black phosphorus. Other physical properties include: Colorless and transparent in its pure form
Waxy white solid
-Insoluble in water, but soluble in carbon disulfide
-Burns spontaneously in air to its pentoxide
-Melting point of 44.1 °C (white), boiling temperature of 280°C (white)
-Density of 1.82 grams per cubic centimeter
CHEMICAL: Phosphorus is a member of the Nitrogen Family and has 5 valence shell electrons available for bonding. Its valence shell configuration is 3s23p3. Phosphorus forms mostly covalent bonds. Any phosphorus rock can be used for the production of elemental phosphorus. Crushed phosphate rocks and sand
Uses for Phosphorus

To make safety matches. "Phossy Jaw" was a condition some people developed after being exposed to phosphorus in match factories

Used as a flame-retardant in textiles, plastics and sealants. Inhaling these can irritate the nose and throat and long term use can affect the liver and kidneys.

White phosphorus which is used in bombs is highly toxic and can cause liver, kidney and heart damage if ingested or absorbed into the bloodstream.

Used as a cleaning agent and water softener, phosphorus can be toxic in our waterways and can be detrimental to humans in large quantities.
Discussions

Dmitri Mendeleev is credited for the organization of the periodic table, however, Glenn Seaborg also had a major influence. He took part in the discovery of ten of the periodic table’s chemical elements which led to the periodic table being rewritten. He also co-discovered technetium-99m, the most commonly used medical radioisotope in the world.
The current official number of elements that can be found on a periodic table is 118.