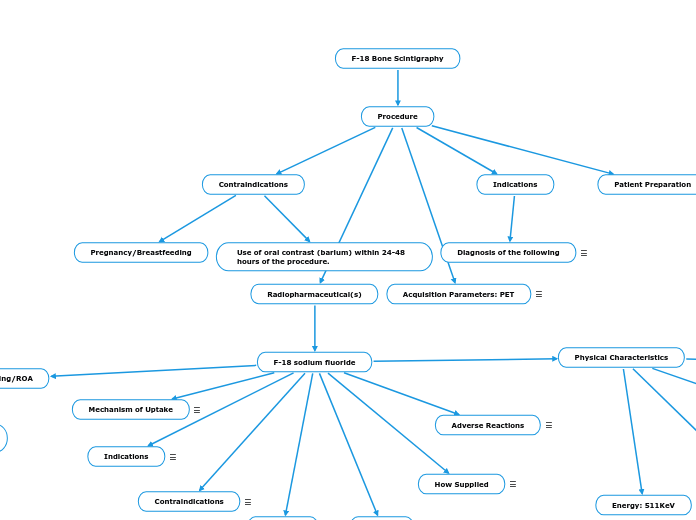
F-18 Bone Scintigraphy
Procedure
Contraindications
Pregnancy/Breastfeeding
Use of oral contrast (barium) within 24-48 hours of the procedure.
Indications
Diagnosis of the following
Patient Preparation
Radiopharmaceutical(s)
F-18 sodium fluoride
Physical Characteristics
Energy: 511KeV
Cyclotron Produced
F18 decays by positron (β+) emission
Half-life of 109.7 minutes
Mechanism of Uptake
Indications
Contraindications
Warnings
Adverse Reactions
How Supplied
Storage
Dosing/ROA
Adult Dose: 5mCi (5-10 mCi) I.V.
Pediatric Dose:0.06 mCi/kg (minimum of 0.5mCi administered) I.V.
Acquisition Parameters: PET