SEPARATION OF MIXTURES

Filtration
Filtration is a process used to separate solids from liquids or gases
Subtopic
The fluid that passes through the filter is called the filtrate.
Method:
Filtration, the process in which solid particles in a liquid or gaseous fluid are removed by the use of a filter medium that permits the fluid to pass through but retains the solid particles. In some processes used in the production of chemicals, both the fluid filtrate and the solid filter cake are recovered.

Simple Distillation
Simple distillation is a procedure by which two liquids with different boiling points can be separated
As the liquid being distilled is heated, the vapors that form will be richest in the component of the mixture that boils at the lowest temperature.
Method: Simple distillation is a procedure by which two liquids with different boiling points can be separated. ... The temperature will continue to increase until the boiling point of the next-lowest-boiling compound is approached. When the temperature again stabilizes, another pure fraction of the distillate can be collected.
Fractional Distillation
Fractional distillation is the separation of a mixture into its component parts, or fractions.
Subtopic
It uses distillation to fractionate.
Method: Fractional distillation is done by heating the mixture so that each fraction evaporates and then condenses in its own compartment. It is a special type of distillation.
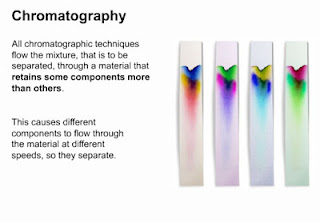
Chromatography
Method: A drop of concentrated solution is placed on a pencil line near the bottom edge of a strip of chromatography paper, the paper is then dipped into the solvent. the level of the solvent must start below the sample.
Chromatography is a laboratory technique for the separation of a mixture.
The various constituents of the mixture travel at different speeds, causing them to separate
:max_bytes(150000):strip_icc()/GettyImages-736992929-5bacf81cc9e77c002cf9e308.jpg)
Decantation
It is a method used in labs to separate mixtures from each other
In simple form it means to separate solid and liquids from each other through the force of gravity
Method:
Decantation is a process to separate mixtures by removing a liquid layer that is free of a precipitate, or the solids deposited from a solution. The purpose may be to obtain a decant (liquid free from particulates) or to recover the precipitate.

Crystallization
Crystallization is the solidification of atoms or molecules into a highly structured form called a crystal
This refers to the slow precipitation of crystals from a solution of a substance.
