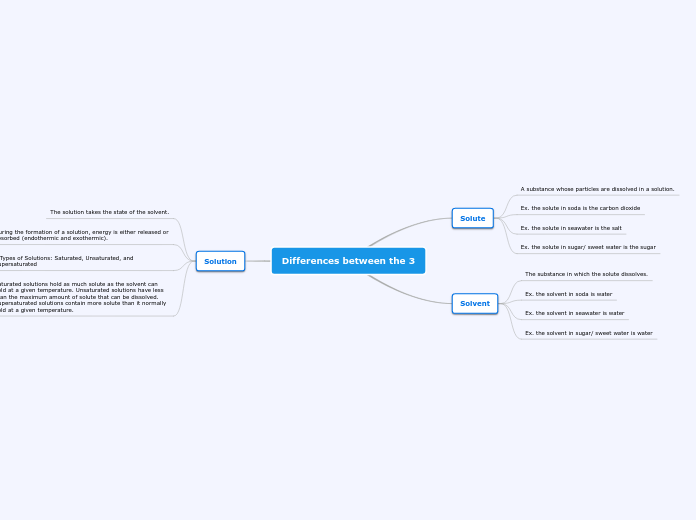
Differences between the 3
Solute
A substance whose particles are dissolved in a solution.
Ex. the solute in soda is the carbon dioxide
Ex. the solute in seawater is the salt
Ex. the solute in sugar/ sweet water is the sugar
Solvent
The substance in which the solute dissolves.
Ex. the solvent in soda is water
Ex. the solvent in seawater is water
Ex. the solvent in sugar/ sweet water is water
Solution
The solution takes the state of the solvent.
During the formation of a solution, energy is either released or absorbed (endothermic and exothermic).
3 Types of Solutions: Saturated, Unsaturated, and Supersaturated
Saturated solutions hold as much solute as the solvent can hold at a given temperature. Unsaturated solutions have less than the maximum amount of solute that can be dissolved. Supersaturated solutions contain more solute than it normally hold at a given temperature.