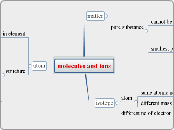
molecules and ions
matter
mixture
can be separated by phy method
not uniform
heterogenous
uniform
homogenous
pure substance
cannot be separated bt phy method
can be decomposed by chem process
element
cannot be decomposed by chem process
compound
can be from by ionic bonding
transfer of electron
element combine chemically
differ from constituent element
constant composition
smallest particle found
molecule
form from covalent bonding
sharing of electron
isotope
atom
same atomic no
different mass no
different no of electron
atom
smallest particle in element
structure
proton (P)
charge +1
electron (E)
charge -1
surround nucleus
nuetron (N)
charge 0
P+E = nucleus
No.P = No. E
No. P+ No.N = mass no
weight average mass of an element