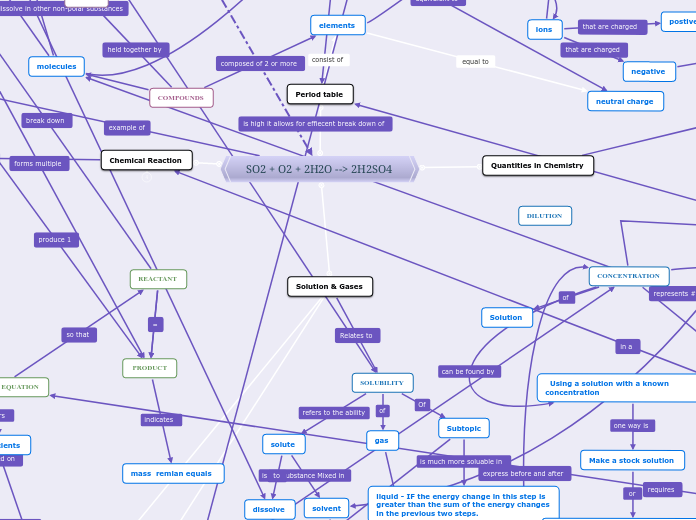
SO2 + O2 + 2H2O --> 2H2SO4
Chemical Reaction
Solution & Gases
Tiration
gas laws
Quantities in Chemistry
Period table
elements
neutral charge
Summary
Number of moles can be found by you use m/M. Molar mass is determined using m/n
share valence electrons
ions
COMPOUNDS
molecules
diatomic
polyatomic
BONDS

covalent bonds

ionic bonds
metals and non metals
POLARITY
Bond polarity
molecular polarity
6 TYPES OF REACTION

synthesis

Decompostion
/methanecombustion-58e3e6005f9b58ef7e0daa10.jpg)
Combustion
Hydrcarbon
Oxygen
Water
Carbon dioxide

double displacement
water
Precipitate
solubility chart

Neutraliation
:max_bytes(150000):strip_icc()/single_displacement_reaction-56a1327a3df78cf7726851ad.png)
Single displacement
a more active element replaces the least active element
Subtopic
BALANCING EQUATION
coefficients
REACTANT
PRODUCT
mass remian equals
SOLUBILITY
solute
dissolve
Temperture
kinetic energy
solvent
solution
supersaturated
more than the maximum amount of solute that can be dissolved in a solvent at a given temperature and pressure
unsaturated
solution in which more solute can be dissolved in the solvent at a given temperature and pressure
Saturated
the maximum amount of solute that can be dissolved in a solvent at a given temperature and pressure
gas
soluable
Subtopic
liquid - IF the energy change in this step is greater than the sum of the energy changes in the previous two steps.
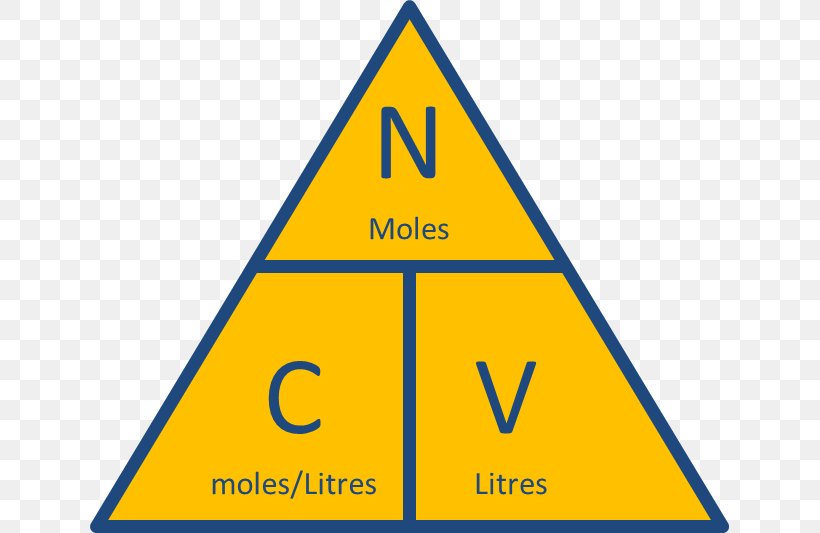
MOLE
Substances
Stoichiometry
Reactants
Product
Limiting reactant
product
Yield
theorotical yield
Actual yield
percen yield

Excess reactant
Coefficient
mole ratio
mol to mol

Final product
percent yield
ACIDS AND BASES
water and salt
acids
base
OH ions
protons
Hydogen ions
protons
PH scale
KINETIC MOLECULAR THEORY
PERIODIC TRENDS
ATMOIC NOTATION
symbol
DILUTION
CONCENTRATION
r^
Percentage by mass
Percent by volume
molar concentration
Using a solution with a known concentration
Make a stock solution
dilute by adding known amount of water
Dilution
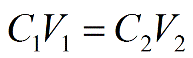
Volume
Solution
FACTOR LABEL METHOD
Units
the ratio of the amount of solute to the
amount of solution
Dilution
concentrated
Isotopes
Atoms
protons
neturons
Radio Isotopes

charge
mass number
atomic number
Atomic Radius
Atom diagram
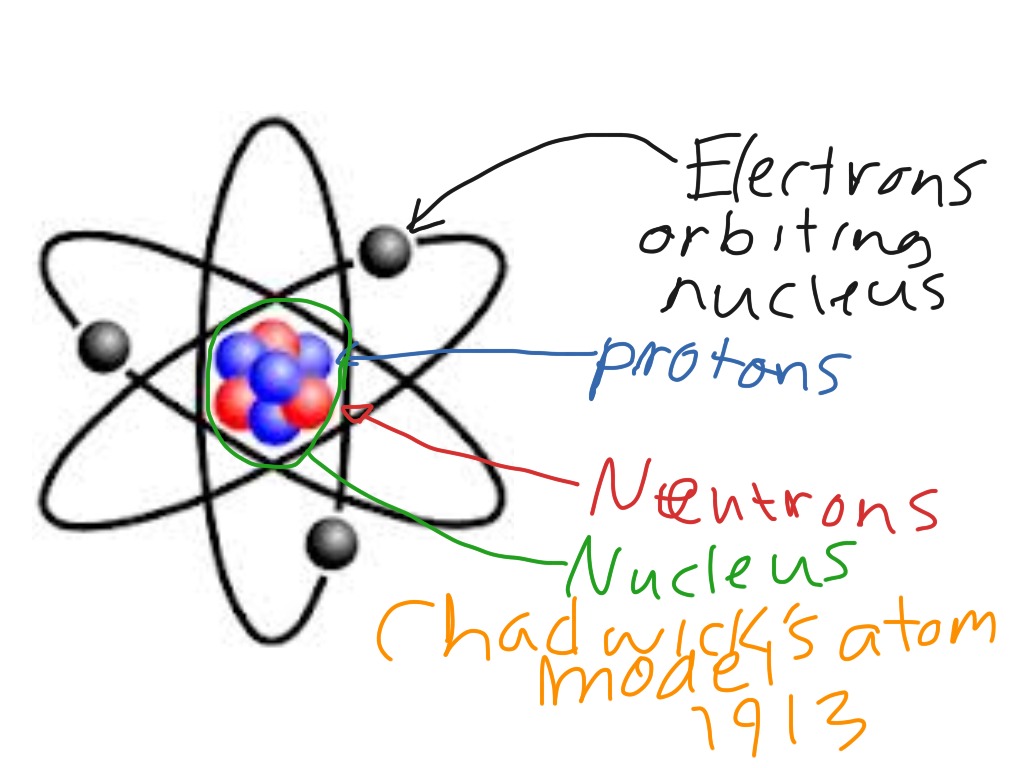
Chadwick model
First Ionization Energy
Electron Affinity
Electronegativity
Reactivity of Metals
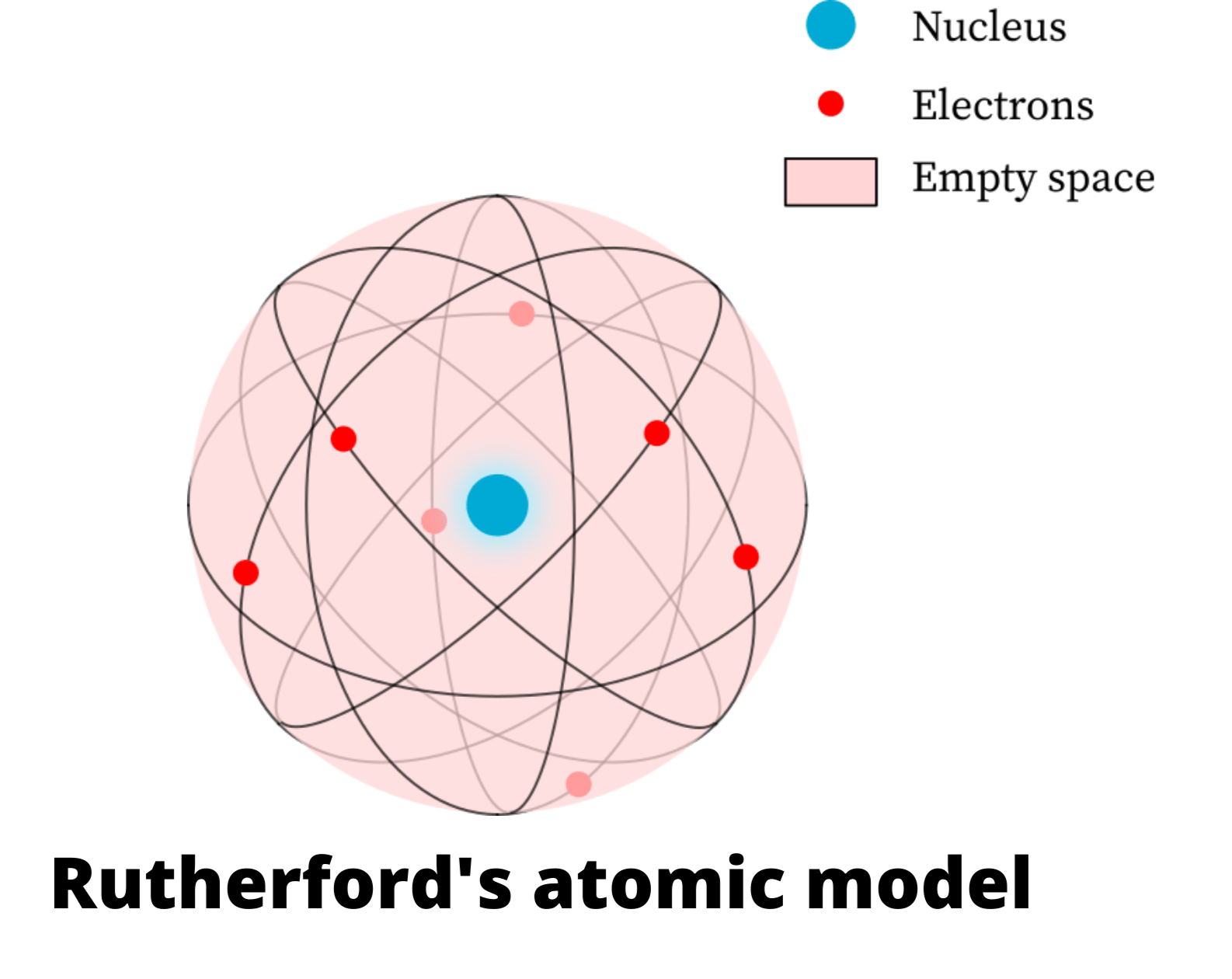
Rutherford’s Model

Thompson Model

Bohr’s Model
Reactivity of Non Metals
Geometry
electron pairs
lone pairs
postive
cations
negative
Anions
∆EN = 0
∆EN >1.7
0 < ∆EN ≤ 1.7
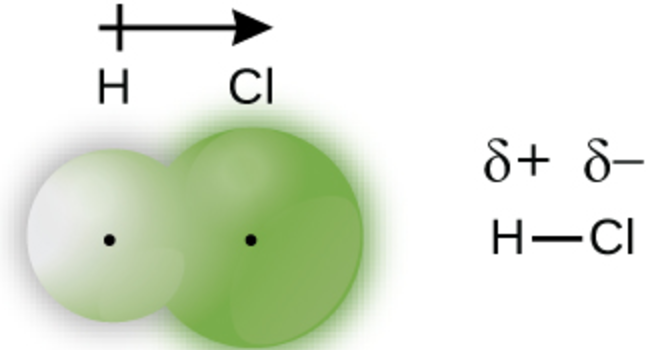
polar
Attach photo of my molarity equation
.PNG)
ionic bond

non-polar
VSPER
Determine whether molecule is
.jpg)
particles
Molar mass
Mass
grams
Mass and mol

Atoms
Molecules
can be calculated of
INTERMOLECULAR FORCES
London dispersion : Electrons are constantly moving. Bunching up causing one negative and the other positive result in a temporary attraction. It will happen with polar and non-polar
Dipole- dipole occurs between one positive end and one negative end.
Hydrogen bonding -a type of dipole-dipole however it happen between hydrogen and either oxygen, nitrogen or flourine
types include
Solubility Curve