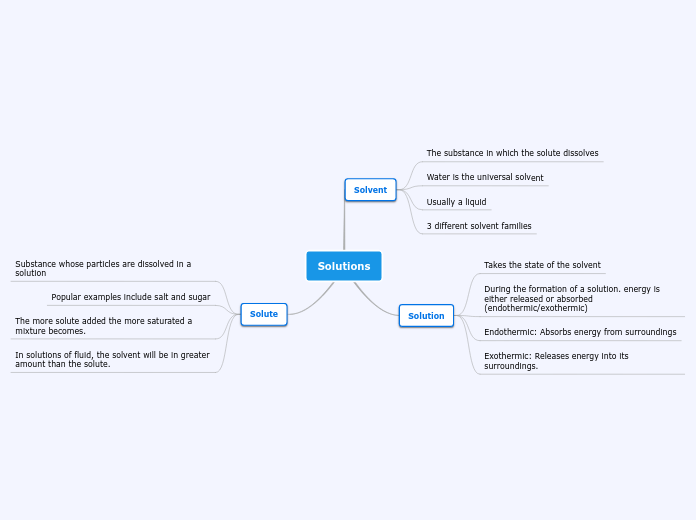
Solutions
Solvent
The substance in which the solute dissolves
Water is the universal solvent
Usually a liquid
3 different solvent families
Solution
Takes the state of the solvent
During the formation of a solution. energy is either released or absorbed (endothermic/exothermic)
Endothermic: Absorbs energy from surroundings
Exothermic: Releases energy into its surroundings.
Solute
Substance whose particles are dissolved in a solution
Popular examples include salt and sugar
The more solute added the more saturated a mixture becomes.
In solutions of fluid, the solvent will be in greater amount than the solute.