By: Maizie Fernandes, Rafael Sanchez, Isabella Penick, Caroline Onwuzu, Syeda Jilani
Proved that DNA was critical in having genetic information.
DNA Structure & Replication Experiments
Messleson and Stahl
Proved that Semiconservative model was accurate to how DNA replication worked.
Proved Conservative and Dispersive model was not accurate to how DNA replication worked.
Hershey-Chase
radioactive phosphorous within bacteriophage DNA did go inside bacterial cell.
radioactive sulfur within bacteriophage protein did not go inside bacterial cell
Griffith Experiment
Smooth heat killed bacteria with rough killed mice
Smooth heat killed bacteria did NOT kill mice
Smooth with rough bacteria did NOT kill mice
Smooth bacteria injected in mice killed mice
Protocell
Evolution in Prokaryotic Cells
Oparin's Bubble Hypothesis
Identified Hydrogen cyanide, formaldehyde, amino acids, hydrocarbons within primitive atmosphere.
Abiotic synthesis of DNA, RNA
Primitive DNA, RNA
Abiotic synthesis of proteins
Primitive proteins
Cyclin dictates the target protein to phosphorylate by the CDK
Meiosis
Makes 4 daughter cells
Starts diploid (2n=46)
n=23
n
Hyperpolarization
Refractory period
Cell Cycle Regulators
CDK (Cyclin-dependent kinases)
structurally and functionally related proteins
Kinases: add phosphate to target proteins
Cyclin
Structurally and functionally related proteins
Gene Regulation
Distal control elements
Can be located in introns
located up or down stream
enhancers
Transcription Factors
Specific
lead to increased expression
Require activators
General
basal levels of transcription
Proximal control elements
Sequences close to promoter
Phases
Prophase: Chromosomes condense,
mitotic spindle forms, nucleolus disappears
Prometaphase: nuclear envelope
releases releases condensed chromosomes,
Metaphase: chromosomes
align at the plate
Anaphase: sister chromatids separate
from each other and are pulled towards
opposite ends of the cell
Telophase: chromosomes decondense,
nucleoli returns, new nuclei are formed
for the 2 daughter cells
Cytokinesis: cytoplasm splits to form new
cells; in plant cells a cell plate forms
that eventually becomes a cell
wall
Meiosis 2
Meiosis 1 (haploid n=23)
Mitosis
Makes 2 daughter cells
diploid 2n=46
2n
DNA Packaging
Nucleosome
Nucleosome: eight histones w/ DNA wrapped around
"Beads on a string"
Looped Domains
compressed fiber
Metaphase Chromosome
Linker DNA
Histone Core: H2A, H2B, H3, H4
in RNA, Adenine (A) pairs w/ Uracil (U) and Thymine is not present.
Linkage of nucleotides
Involves dehydration reaction
This creates the sugar-phosphate backbone
Bulk Transport
Endocytosis
phagocytosis
pinocytosis
receptor-mediated endocytosis
Exocytosis
Process of selectively amplifying a piece of DNA
Step 3: Elongation
Mimics DNA Pol III by the starting of Taq polymerase
Step 2: Annealing (attach)
Mimics primase by primers attaching to template DNA
Step 1: Denaturation (helicase)
Heating DNA to separate strand
Active Transport
Moves solute against conc. gradient
Mutations
Types of Mutations
Frameshift
Ex: if 3 codons are added, NOT known as frameshift
Ex: if 1 or 2 codons are added, known as frameshift
Insertion or deletion
Silent
No amino acid change, just change of nucleotide
Nonsense
Any amino acid that stops prematurely
Missense
ex: Glu -> Ala
1 amino acid change
Vesicles
Transport to Golgi
Secrete out
depart of membrane
Extracellular membrane
Can go to back to ER or Lysosomes
Translocation
ER signal
4. Glycosylation: adding sugar groups to protein
will now be a glycoprotein
3. Signal peptidase cuts protein
2. goes to ER and SRP comes off
1. Signal Recognition Protein (SRP) attaches and pauses
Integral
embedded into membrane
Ribosomes
rRNA
E site
tRNAs leave the ribosome from here.
A site
holds tRNA carrying the next amino acid to be added to the chain
P Site
holds tRNA carrying the growing polypeptide chain
Polymerase Chain Reaction (PCR)
Biological molecules
Nucleic Acids
Composed of monomers called nucleotides
Three phosphate groups
Nitrogen-containing (nitrogenous) base
Types of Nitrogenous bases
Purines: Adenine (A), Guanine (G)
Pyrimidine:
Cytosine (C), Thymine (T), Uracil (U)
Five carbon sugar (pentose)
In RNA, Sugar is Ribose
In DNA, sugar is Deoxyribose
Proteins
Denaturation: when transferred from aqueous to non-polar
Structures
Quaternary
Tertiary
Secondary
Primary
Amino Acid
R Groups
Peptide bonds
PH 7.2
Lipids
Testosterone
Cholesterol
Fatty acid
Ester linkage
Tryglyceride
Phospholipds
Phosphate Bi-layer
hydrophobic tails
hydrophilic head
unsaturated
double bonds
saturated
no double bonds
Carbohydrates
Plant starch
Amylopectin
Amylose
Disaccharide
Polysaccaride
Structure
chitin
cellulose
Storage
dextran
starch
glycogen
Glucose
Aldo and Keto
ALPHA AND BETA
Glucose + Fructose=Sucrose
Glucose + Glucose= Maltose
Hydrolysis
Breaks down polymers
Dehydration
Van der Waals
electrons not evenly distributed causing them yo stick together
non-polar bonds have + and - charges regions
when atoms are close, weak interactions
Hydrogen bonds
Cohesion
Adhesion
heat absorbed hydrogen bonds break, heat released when they reform
If they don't reform there is evaporation
when hydrogen atoms are covalently bonded to electronegative atom
EX: water molecule connections
strong dipole dipole interactions
Synapses
Electrical Synapses
enable direct flow from neuron to neuron
gap junctions
Chemical Synapses
increase in Ca+ causes vesicles to fuse w/ pre-synaptic membrrance
relies on pre-synaptic neuron to release neurotranmitter
Action Potential
Constant magnitude
Undershoot
Some K+ open; Na+ closed
Returns to resting potential
Falling Phase
Inside of the cell negative
Na+ inactivated; K+ open; K+ outflow
Rising Phase
Inside of cell more positive
Most Na+ open; Na+ influx
Depolarization
If threshold reached, AP begins
Some Na+ open
Resting State
Na+ and K+ closed
ATP produced
Cell Cycle
Interphase
G1: Cell prepares to duplicate
S: Chromosomes duplicated (46)
Sister chromatids form
G2: Cell checked for errors
Membrane potential
Positive outside of membrane
Electrochemical grandient
Electrogenic pump
Proton Pump
Cotransport
H+/Sucrose Pump
Negative inside of membrane
Anions out
Cations in
Facilitated Diffusion
Ion Channels
Not gated
open/close in response to shift in voltage
Gated
open/close in response to stimuli
Tryptophan
repressor= inactive; tryptophan (co-repressor) needed to bind to operator
Co-repressor
works w/ repressor to turn operon off
Carrier Proteins
Sodium-Potassium Pump
Ion channels
Exchanges NA+ with K+
Lactose
Prokaryotes
Operon
LAC I
Promoter
Operator
LAC Z (Beta Galactosidase)
LAC Y (Permease)
LAC A (Transacetylase)
Terminator
Regulatory gene
Inducer turns LAC I off
Negative Regulation
Repressor= no transcription
Positive Regulation
activator= transcription
basal level expression at all times
Transmembrane proteins
Passive Transport
Osmosis
Diffusion
High conc to Low Conc
Channel Proteins
aquaporins
Cell Signaling Pathways
phosphodiester bonds
Cancer
Tumor-suppressor genes
encode proteins that inhibit abnormal division of cells
BRCA 1 and BRCA 2
breast and other tissue
help repair damaged DNA
mutation: p53
Characterisitics of cancer cells
ability to invade/disrupt distant and local tissues
accumulation of mutants
loss of anchorage dependence
loss of density dependent inhibition
proto-oncogenes
normal genes that stimulate normal cell growth/division
RAS
oncogenes
cancerous genes
Eukaryotes
G-Protein Signaling
hormone binds to receptor
receptor changes shape (receptor activated)
G-Protein activated
G-Protein binds to receptor
GDP ---> GTP
hormone leaves receptor
Adenylyl Cyclase= enzyme
Adenylyl Cylclase activated
ATP -->cAMP
GTPase
GTP---> GDP
Adenylyl Cyclase inactivated
Protein Kinase A = enzyme
cAMP bind to Protein Kinase A
phosphorylation cascade
Response
Cell-Cell Recognition
Phospholipid bilayer
Steroid Cholesterol
"fluidity buffer"
Unsaturated tails
Remains fluid
Tonicity
Isotonic
Normal
Flaccid
Hypotonic
Bursts
Turgid
Hypertonic
Lyse
Plasmolyzed
DNA Translation
mRNA
Transfer RNA (tRNA)
Amino acyl tRNA synthetase adds amino acid to tRNA.
reads codon message from mRNA to transfer amino acid to grow polypeptide
Makes: mRNA
Has terminator sequence
No Splicing
Makes: Pre-mRNA
RNA Splicing
Introns: spliced out
Exons: stay in mRNA
No terminator sequence
DNA Transcription
DNA to RNA
Eukaryotes: RNA Polymerase II
Prokaryotes: RNA Pol I
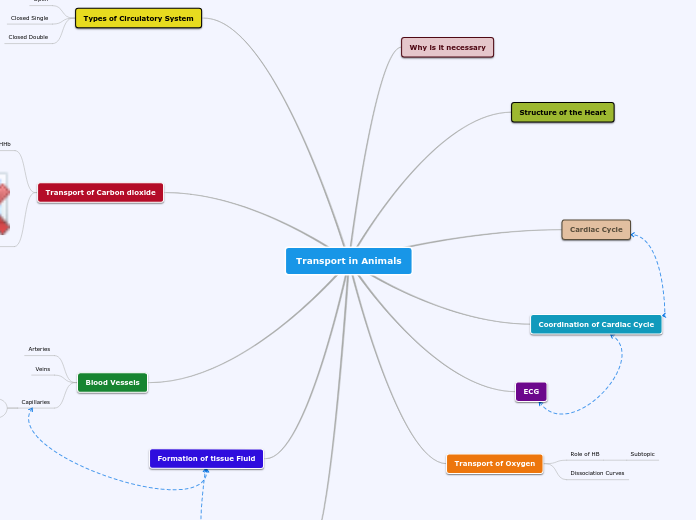
Single Strand Binding Protein
Topoisomerase
Helps reduce tension between ends of uncoiled DNA
6. DNA Ligase
Helps form phosphodiester bonds between DNA
5. DNA Pol III
Lagging Strand: elongate new strand in 5 to 3 direction. Works away from replication fork.
Leading Strand: synthesizes a complimentary strand continuously by elongating new DNA. (5 to 3 direction)
4. DNA Pol I
Has proofreading abilities (3 to 5 direction)
Synthesize new DNA by adding of nucleotides (5 to 3 direction)
3. Primase
makes short segment of RNA
adds RNA nucleotides one at a time
2. Primase adds RNA nucleotides
multiple primase for lagging strand
1.Helicase
Unwinds DNA
DNA Replication
starts w/ ORI at DNA
Endosymbiotic theory
No Membrane Enclosed Organelles
DNA in nucleoid
Organelles
Cytoplasm
Cytoskeleton
Intermediate Filaments
Made up of keratin
Make up nuclear lamina; lines up in nuclear envelope.
Specialized for bearing tension
Microfilaments
Also known for having myosin; muscle
contraction cells.
found inside plasma membrane
Made up of actin proteins
Function: bear tension (pulling forces of ECM)
Microtubules
Ex: movement of cilia and flagella
Each tubulin is a dimer; composed
of two polypeptides.
Globular proteins called tubulin
Peroxisome
formation of H2O2 from reaction; then it is separated
into H2O and O2
DNA Within Nucleus
Mitochondria
site for cellular respiration; Oxygen used to make ATP
Chloroplast
Plants and Algae; site for photosynthesis
Biology Concept Map
Energy, Cellular Respiration, Photosynthesis
Photophosphorylation
ETC --> diffusion --> ATP
Calvin cycle
phase 3: generating a carbon receptor
phase 2: reduction
phase 1: carbon fixation
ATP and NADPH used
Light Reactions
SPLIT H2O, RELEASE O2, PRODUCE ATP, FORM NADPH
cyclic vs non-cyclic
light absorption: ground state --> excited state = unstable
porphyrin ring + hydrocarbon tail
6CO2+6H20+ENERGY--->C6H12O6+6O2
Oxidative phosphorylation
Chemiosmosis
Electron transport chain
Citric acid cycle
ETC
Glycolysis
Energy investment
ATP used to make ADP
fructose 6-phosphate --PHOSPHOFRUCTOKINASE--> F 1,6-bisphosphate
glucose --HEXOKINASE--> G 6-phosphate
Energy payoff
Pyruvate formed
Lactic Acid fermentation
Alcohol fermentation
Energy
Energy Coupling
uses ATP
Free Energy
Spontaneous change
chemical reaction
endergonic
exergonic
diffusion
gravitational motion
Potential
Kinetic
Metabolism
Anabolic
Photosynthesis
Catabolic
Respiration
Substrates
active site
Inhibitors
Noncompetitive
Competitive
Test 1
Organic Molecules
Functional groups
phosphate group
amino group
carboxyl group
carbonyl group
hydroxyl group
CARBON
covalent compatible with many different elements
Isomers
Enantiomers
asymmetric carbon
Geometric
cis-trans isomers
Subtopic
Structual
different covalent arrangements
Carbon skeletons: 4 WAYS
Presence of Rings
Double bond position
Length (branched and unbranched)
Hydrocarbons
Water and its properties
PH Scale
[H+] [OH-] = 10 ^-14
Human body is 7.4, slightly basic
Buffers maintain a constant PH
Base (-)
Acid (+)
Water expands when it solidifies
water & sugar= MIXTURE
water & salt= SOLUTION
Ice is 10% LESS dense, lighter than liquid
Evaporative cooling
Amphipathic
Hydrophobic and Hydrophillic
High specific heat
Chemical Bonding
Polar covalent bonds
Hydrophillic
formed by two opposite elements
O-H, O-C, O-O
Ionic compounds
formed by two elements of opposite charges
Anions (-)
Cations (+)
electrons are NOT shared
NOT as strong as covalent bonds in BIOLOGY
Nonpolar covalent bonds
Hydrophobic
formed by two of the same elements
C-H, C-C, O-O
Covalent bond
sharing of a pair of valence electrons by two atoms
Golgi bundles the vesicles and the vesic;es spill contents out of the plasma membrane
Cell Signaling Pathways and Cancer
Test 2
Eukaryotic Cells
Plants
Animals
Fungi
Protists
Prokaryotic Cells
Bacteria
Archaea
Extreme Thermophiles
Methanogens
Extreme Halophiles