ATOMIC MODELS
SCHRÖDINGER(1926)
Describe the motion of electrons as standing waves
The model does not contemplate the stability of the nucleus, it only refers to explaining the quantum mechanics associated with the movement of electrons within the atom.
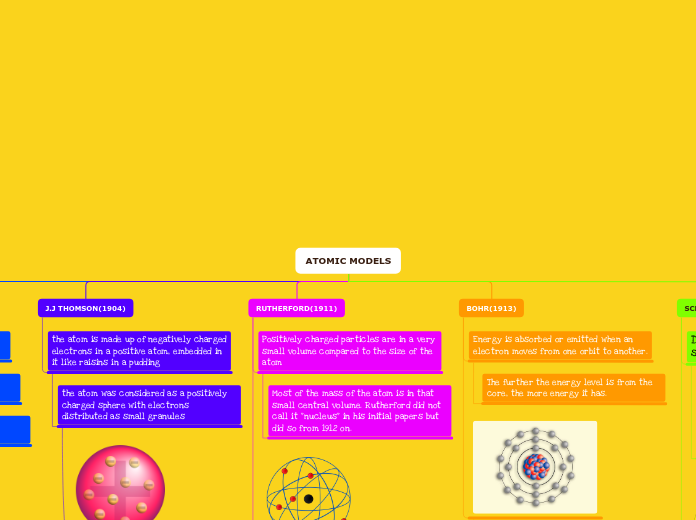
BOHR(1913)
Energy is absorbed or emitted when an electron moves from one orbit to another.
The further the energy level is from the core, the more energy it has.
RUTHERFORD(1911)
Positively charged particles are in a very small volume compared to the size of the atom
Most of the mass of the atom is in that small central volume. Rutherford did not call it "nucleus" in his initial papers but did so from 1912 on.
J.J THOMSON(1904)
the atom is made up of negatively charged electrons in a positive atom, embedded in it like raisins in a pudding
the atom was considered as a positively charged sphere with electrons distributed as small granules
DALTON MODEL(1803)
Dalton proposes indivisible unit of an element is the atom
atoms can combine to form chemical compounds
atoms do not have division, even in chemical reactions