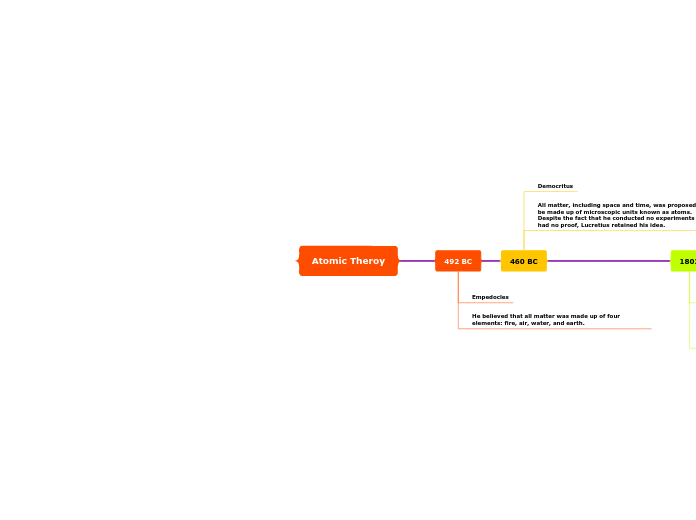
Atomic Theroy
492 BC
Empedocles
He believed that all matter was made up of four elements: fire, air, water, and earth.
460 BC
Democritus
All matter, including space and time, was proposed to be made up of microscopic units known as atoms. Despite the fact that he conducted no experiments and had no proof, Lucretius retained his idea.
1803
Dalton
Dalton agreed that atoms, which he believed were indestructible, generated all matter. Also, compounds are formed by combining two atoms, and all atoms of a specific element have equal mass attributes (Dalton's atom Theory).
1869
Mendeleev
He developed the periodic table of elements, which arranges elements based on similarities. His Period Law asserts that "physical and chemical properties of elements are periodic functions of their atomic numbers."
1885
Eugene Goldstein
Positive particles were discovered. Taking into consideration that the particles had a charge of equal and opposite to the electron
1896
Henri Becquerel
He was awarded the Nobel Prize for discovering radioactivity. It was also discovered that uranium rays caused gases to ionize.
1897
J.J. Thomson
He was the one who discovered the electron. He tested and studied the nature of electric discharge in a high vacuum cathode-ray tube.
1907
Ernest Rutherfor
He discovered the present model of an atom using the gold foil experiment. He came to the conclusion that all positive charge was centred, and negative electrons orbited the nucleus.
1910
Millikan
He determined the charge of a single electron using an oil drop experiment.
1913
Neils Bohr
He explained that an atom's outer orbits might hold more electrons than its inner orbits. Knowing this allows one to calculate the chemical characteristics of an atom. He also proposed that electrons emit light by hopping orbits.
Fredrick Soddy
He invented the notion of isotopes. Explain to Ernest Rutherford that radiation is caused by elemental transmutation.
1925
Heisenberg
He thought of the Uncertainty Principle. According to the principle, one can never know the exact position and energy of an electron at the same time.
1932
Chadwick
He was the one who discovered the neutron. The neutron aids in the balance of protons in an atom's nucleus.