Seperating techniques
Filtration
Used to seperate insoluble solids from liquids
A filter paper is used to seperate the insoluble solids from the liquids. The filter paper contains small pores that allows liquid and soluble solids to pass through but not the insoluble solids.
The solid trapped by the filter paper is called the residue.
The substance that passes through is called the filtrate.
Crystallisation
Used to seperate soluble solids that decompose when heated from liquids.
Example: sugar from water; copper sulfate from water.
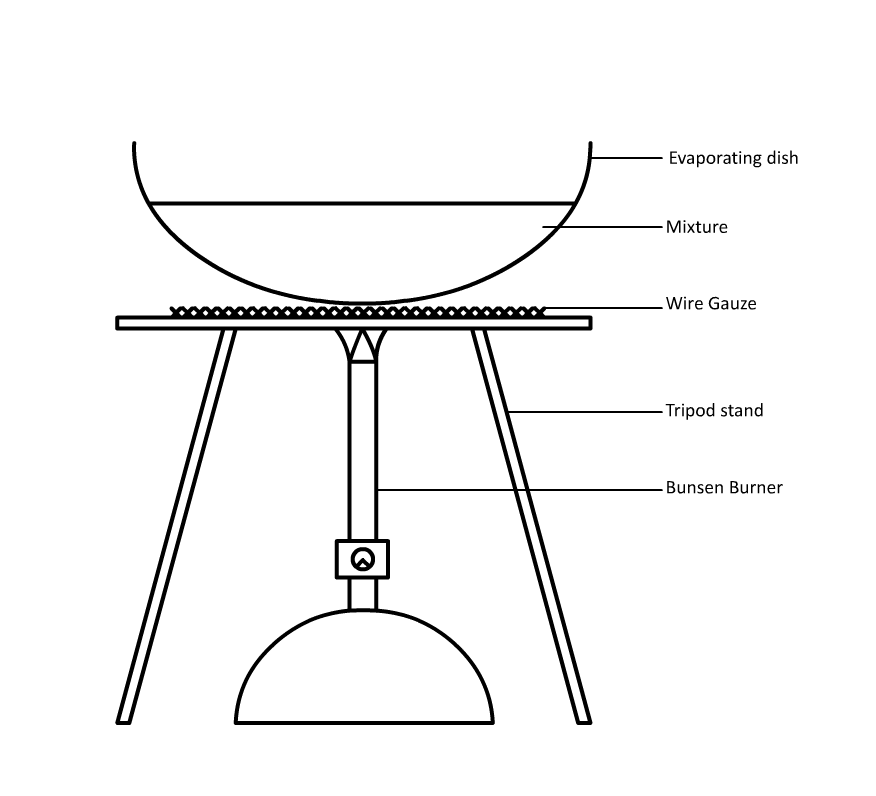
Evaporation
Used to seperate soluble solids from liquids.
To obtain the solid, the solution is evaporated to dryness and the solid is recovered.
This method can only be used for solids that do not decompose when heated.
Example for solids that decompose: Sugar ; Copper(II) sulfate.
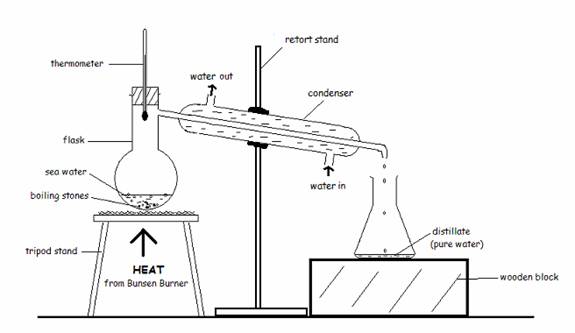
Simple distillation
Used to obtain liquid from solution.
Example:To obtain water from saltwater,seawater.
Magnetic attraction
A magnet can be used to seperate magnetic substance from non-magnetic substance.
Example of substances with magnetic properties: Iron, steel, nickel, cobalt.
Paper Chromatography
Paper chromatography is used to seperate coloured components in a mixture.
Example: Different coloured dyes in black ink, seperate dyes in food colouring , drugs in urine
How does chromatography work?
For paper chromatography to work, the dye must be soluble in the solvent.
Example of solvent: water, ethanol.
The solvent in the test-tube moves up the paper.
When it reaches the spot of the dye, it dissolves the substances in the dye.
As it continues to move, the different substances in the dye move along with it but at different speed.
The one that is more soluble moves the fastest reaches the edge of the paper first.
The one that is less soluble moves the slowest may reach only the middle of the paper.
Due to the different rates of moving through the paper, the various dyes seperate out from one another.
Substances that are oure have only one spot while the substances that are not pure have more than one spot.

Subtopic


If two liquids can mix together, they are known as liquids that are miscible
Fractional distillation is used to seperate the liquids.
The liquids are able to be seperated as they have different boiling points.
Liquid with the lower boiling point will be collected.
Boiling chip must be present to smoothen the boiling process.
During the process of fractional distillation, the mixture of liquids boils and may distil together
Vapour of the liquid with higher boiling point condense along the fractionating column and re-enter the flask where the liquids are being boiled.

The liquid with the higher density will sink to the bottom of the seperating funnel and can be removed by turning on the tap.
Seperating liquids that are miscible
Seperating liquids that are not miscible
If two liquids cannot mix together, they are known as liquids that are immiscible.
Example: oil and water
To seperate these liquids, we use a seperating funnel.


Steps to be taken
Gently heat the solution until most of the water is evaporated.
A saturated solution is obtained.
Allow the heated solution to cool and crystals are formed gradually.
Filter to obtain the crystals.
Dry the crystals between two pieces of filter papers.
Seperating liquids