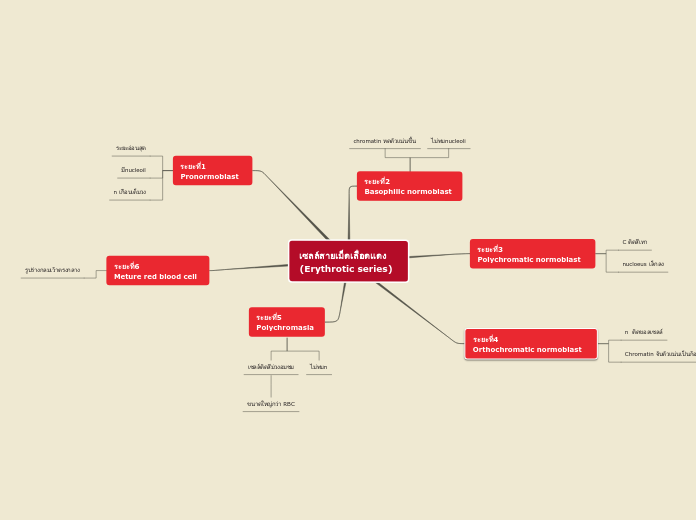
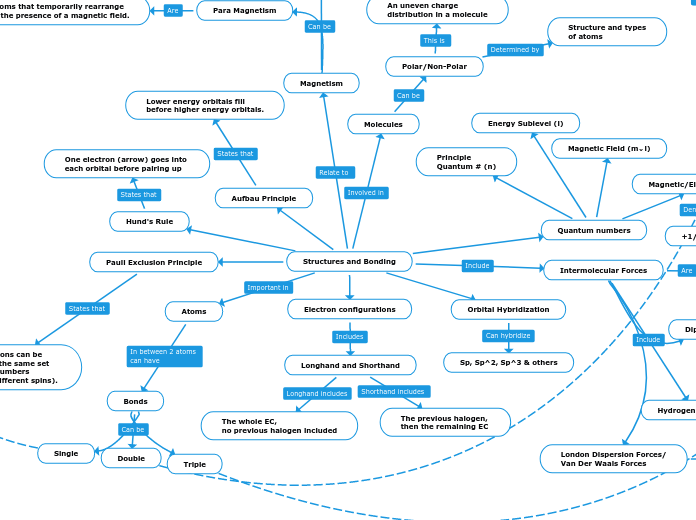
af Kyree Long 6 år siden
276
Chemistry
Atoms are composed of a nucleus containing protons and neutrons, surrounded by an electron cloud. The proton count defines the atomic number, crucial for identifying elements. Neutrons contribute to the mass but vary in number, resulting in isotopes when they differ while protons remain consistent.