af Ummi amira 4 år siden
322
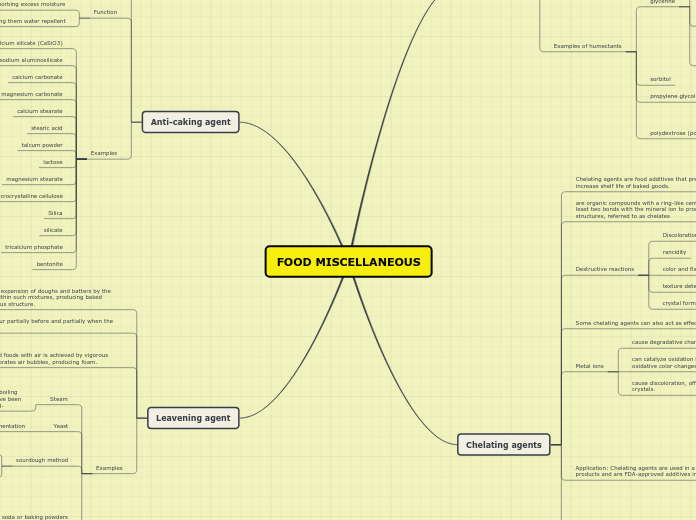
FOOD MISCELLANEOUS
Chelating agents are crucial in food preservation and are approved by the FDA for use in a variety of products, including pickled vegetables, salad dressings, and carbonated beverages.
af Ummi amira 4 år siden
322

Mere som dette
Problem
Release gas at once
if the cake batter sits around for a while the leavening will be lost and your baked goods will be flat.
When it is mixed with an acid liquid it releases the gas carbon dioxide (CO2).
release of carbon dioxide
produce expansion
When added to a fresh dough mixture, the sponge produces fermentation
sugar-fermenting bacteria have been allowed to develop
initiates fermentation
which releases carbon dioxide gas and substances that affect the flavor and aroma of the baked product.
During baking, as the interior of the product nears the boiling point, the vapor exerts pressure within bubbles that have been incorporated earlier by other means, producing swelling.
Serves the capacity to slow the rate of discoloration as well as preserve aroma. Utilized as a preservative in fruit fillings of pastries and other baked items.
Functions as a chelating agent preventing oxidation caused by metal ions.
increased water retention capacity within the baked item leading to increased moisture and longer shelf life.
During fermentation, leavening enables rise in the product due to the formation of carbon dioxide gas creating air pockets within the crumb as well as the evaporation of alcohol.
In baking, the primary function of disodium pyrophosphate is that the compound serves as a source of acid to react with baking soda resulting in leavening.
The most universally known chelating agent, EDTA serves as a multi-dentate molecule because it can form two different bonds.
By binding metals, chelating agents can delay/retard these activities, thus preserving the functional and sensory properties of food products
reduce the water molecules from migrating
Lowering the Aw
lowering the vapor pressure of a food
propylene glycol is slightly bitter
the choice for more strongly flavored products
Glycerin has a sweet taste
Lowering the vapor pressure of a food
help fruit pieces and flavored ribbons to remain soft in frozen desserts instead of turning hard and icy.
depresses the freezing point.
helps retain moisture
used as a humectant is in dried fruits
raisins
figs
apricots
attracts water and reduces its availability to migrate - a feature that has many uses in foods
offer the advantage of not increasing viscosity in applications where it isn't wanted.