af Raneem Abouseta 6 år siden
517
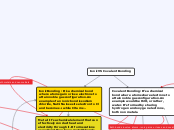
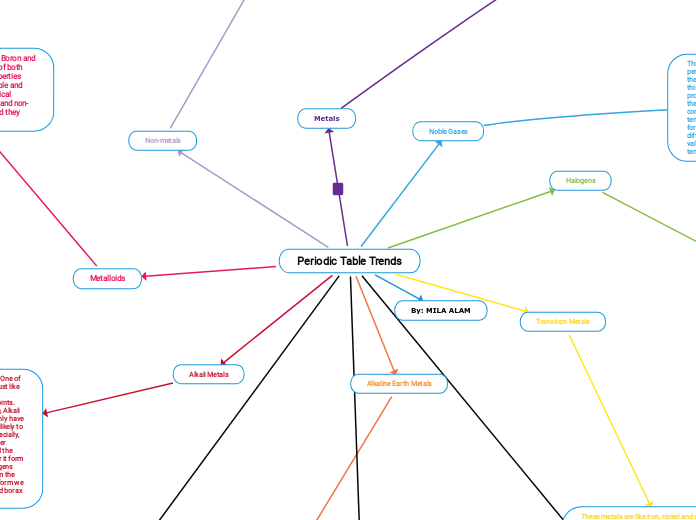
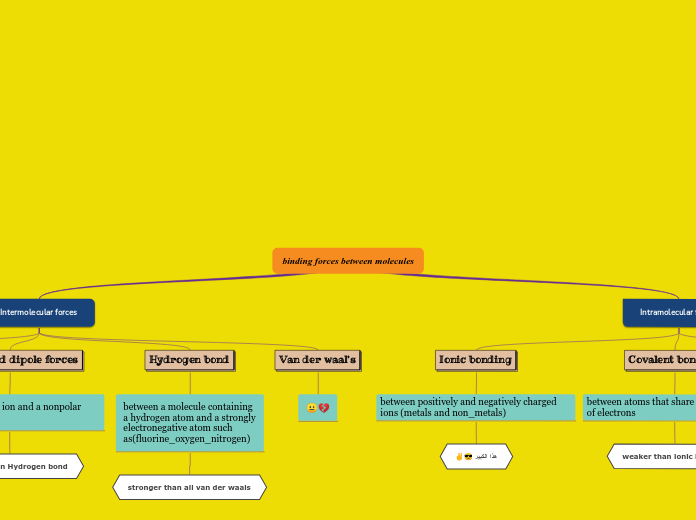
Ionic VS Covalent Bonding
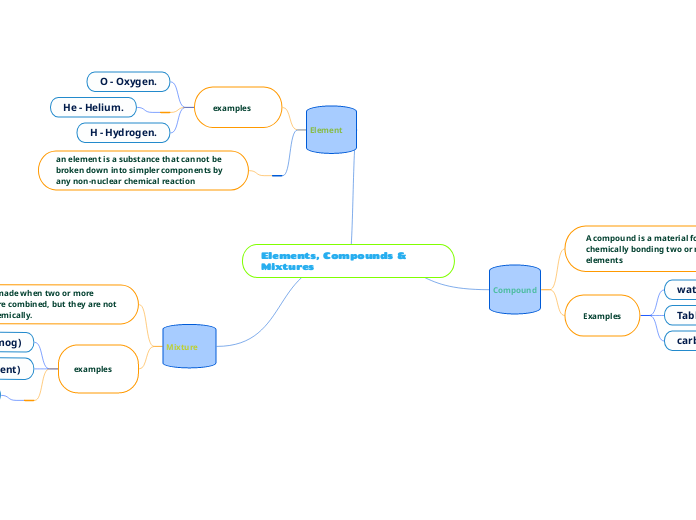
Lewis symbols and structures are visual representations that show the valence electrons in an element or the bonding between atoms in a molecule, such as in methane. Electronegativity is an essential concept that describes an atom'