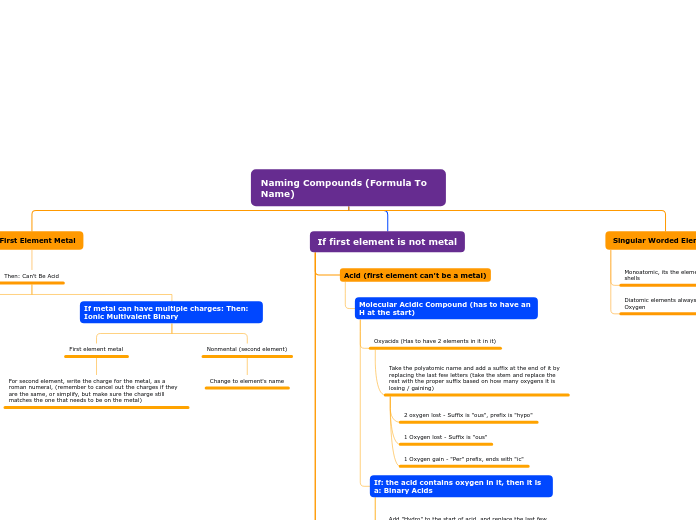
Naming Compounds (Formula To Name)
Singular Worded Elements
Diatomic elements always have a subscript of 2 eg. O2 = Oxygen
Monoatomic, its the element straight, they have full valence shells
If first element is not metal
Molecular Compounds
First element stays the same
If second element ends with a 1:
Start second element with prefix "Mono" and suffix "ide"
If second element ends with a 2:
Start second element with prefix "Di" and suffix "ide"
If second element ends with a 3:
Start second element with prefix "Tri" and suffix "ide"
If second element ends with a 4:
Start second element with prefix "Tetra" and suffix "ide"
If second element ends with a 5:
Start second element with prefix "Penta" and suffix "ide"
If second element ends with a 6:
Start second element with prefix "Se" and suffix "ide"
If second element ends with a 7:
Start second element with prefix "Hept" and suffix "ide"
If second element ends with a 8:
Start second element with prefix "Octa" and suffix "ide"
If second element ends with a 9:
Start second element with prefix "non" and suffix "ide"
Polyatomic Compounds (first element can't be a metal)
Monovalent
Second Element (Polyatomic)
Look for polyatomic compound on the polyatomic chart
If: the subscript does match the original polyatomic element
Keep it the same as no oxygens are getting lost/gained
If: the subscript does not match the original polyatomic element because there are too many oxygens/ too less oxygens
Add the necessary prefix, and suffix that is needed into the polyatomic compound's name e.g.
+1 oxygen pernitrate
root nitrate
-1 oxygen nitrite
-2 oxygens hyponitrite
First Element
Name stays the same
Multivalent
First element stays the same, except the charge, in which you need to swap the charges, and the write the proper change as a roman numeral infront of the first element
Acid (first element can't be a metal)
Molecular Acidic Compound (has to have an H at the start)
If: the acid contains oxygen in it, then it is a: Binary Acids
Acid Bases: Multivalent Metal + Hydroxide
Add "Hydro" to the start of acid, and replace the last few letters with "ric" or take the stem and put "ric" at the end of the name, and add acid at the end of the name
Oxyacids (Has to have 2 elements in it in it)
Take the polyatomic name and add a suffix at the end of it by replacing the last few letters (take the stem and replace the rest with the proper suffix based on how many oxygens it is losing / gaining)
1 Oxygen gain - "Per" prefix, ends with "ic"
1 Oxygen lost - Suffix is "ous"
2 oxygen lost - Suffix is "ous", prefix is "hypo"
If: First Element Metal
Then: Can't Be Acid
If metal can have multiple charges: Then: Ionic Multivalent Binary
First element metal
For second element, write the charge for the metal, as a roman numeral, (remember to cancel out the charges if they are the same, or simplify, but make sure the charge still matches the one that needs to be on the metal)
Monovalent Ionic Binary
Nonmental (second element)
Change to element's name
Metal (First element)
Metal stays the same