von THOMAS JENKINS Vor 3 Jahren
185
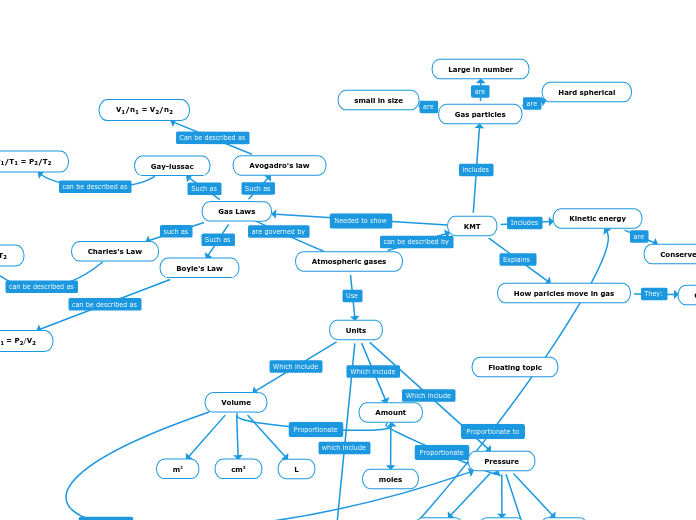
Atmospheric gases
The behavior of gases can be understood through various laws and principles that relate pressure, volume, temperature, and the amount of gas in a system. The Ideal Gas Law is a fundamental equation that helps in predicting the behavior of ideal gases under different conditions.