von Mackenzie Kay Vor 7 Jahren
239
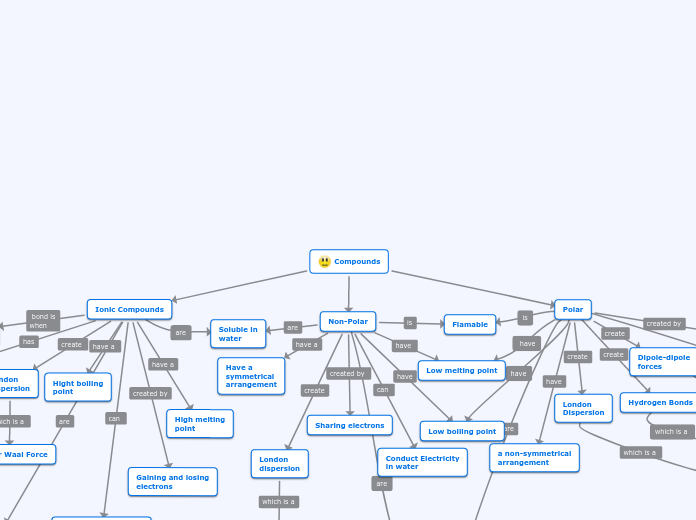
Compounds
In chemistry, compounds can be broadly categorized into ionic, polar, and non-polar types, each exhibiting distinct properties. Ionic compounds form through the transfer of electrons between metals and non-metals, resulting in high melting and boiling points, and the ability to conduct electricity when dissolved in water.