von Sydney Mitchell Vor 5 Jahren
249
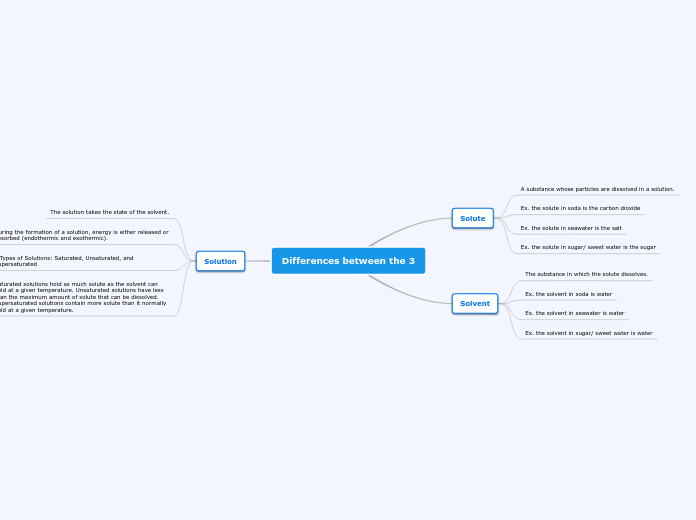
Differences between the 3
Solutions can be classified based on the amount of solute they contain relative to the solvent's capacity to dissolve it. A saturated solution has dissolved the maximum amount of solute possible at a given temperature, while an unsaturated solution contains less solute than it has the capacity to dissolve.