von prajakta sukale Vor 4 Jahren
1005
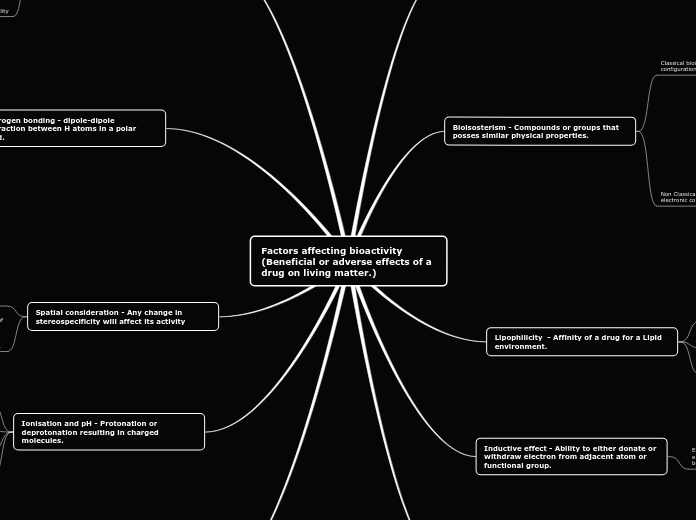
Factors affecting bioactivity (Beneficial or adverse effects of a drug on living matter.)
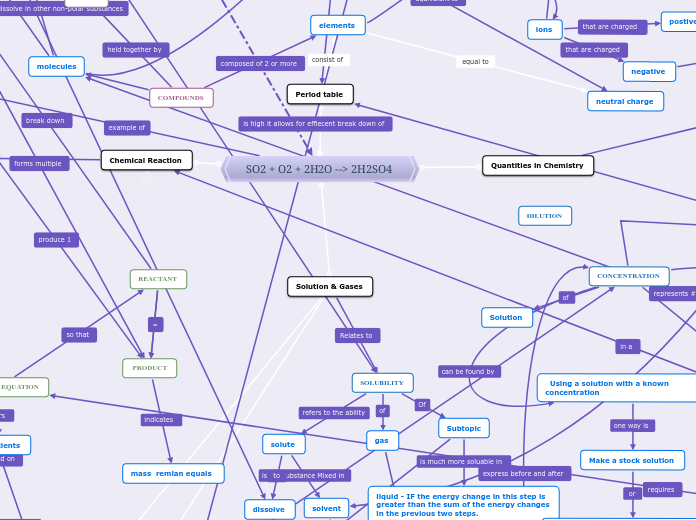
The bioactivity of a drug, which refers to its beneficial or adverse effects on living matter, is influenced by several key factors. Solubility is critical, as a drug must be in solution form to be absorbed and exert its biological effects.