Introduction and Overview
Pharmacokinetic models
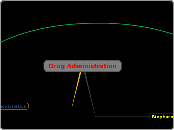
The type of model and route of administration influence which equation is used to characterize plasma concentration versus time data
Compartment model
Types
Three or more compartments
Two compartment
Since there are three phases, a two compartment model with
Three phases
Beta (β) post-distribution phase
Alpha (α) distribution phase
Absorption phase
Since there's a two exponent equation and two phases, a two component model is needed
Cp = A * e ^ -αt + B * e ^ -βt
Terms
B & Beta (β) = associated with drug after distribution
A & Alpha (α) = associated with drug distribution
Since there's two phases, a two exponent equation is needed
Two phases, alpha (α) and beta (β)
Beta phase is after some time when the line becomes straight and distribution equilibrium has been reached
Alpha phase is the first part of the curve (curvilinear) and is where the drug is being distributed in the body
One compartment
Extravascular
Intravenous bolus
Plasma concentration (Cp) versus time (SL)
One compartment is appropriate because there is a single phase in the concentration versus time plot and one exponential term in the equation
Cp = (Cp)₀ * e ^ -Kt
(Cp)₀ = plasma concentration at time = 0
Cp = plasma concentration at any time, t
The straight line also suggests distribution is instantaneous
IV administration means there's no absorption phase
The straight line indicates there's only one pharmacokinetic phase, in this case, elimination
Use and selection
The selection of a compartment model depends solely upon the distribution characteristics of a drug following its administration
Depends on availability of plasma concentration versus time data
Physicochemical properties of a drug
How sensitive the analysis of concentration in plasma is
How often plasma samples are taken
The terms rapid and slow distribution refer to the time required to attain distribution equilibrium for the drug in the body
Distribution equilibrium
When distribution equilibrium is attained, the rate of transfer between the blood and tissue and vice versa is equal
Slow distribution
Suggests that the vasculature, tissues, and organs are not behaving the same way towards the drug
The body must be considered as two or more compartments
In this type of model, the rate of drug transfer from compartment 1 to 2 and vice versa becomes equal at at time greater than zero (from several minutes to hours)
Compartments
The tissues that are not highly perfused like the bones, cartilage, fatty tissue, and many others can be pooled together as one compartment
Highly perfused systems like the liver and kidneys may be pooled together with the blood in one compartment
Suggests the distribution equilibrium is attained slowly and at a finite time (from several minutes to a few hours, depending on drug)
Generally, the slower the drug distribution characteristics of a drug, regardless of the route of administration, the greater the number of compartments required to characterize the plasma concentration versus time data and the more complex the nature of the equation employed
Rapid distribution
All organs and tissues are behaving similarly towards the drug
Suggests the rate of transfer between blood and tissues reaches equilibrium instantaneously after administration
If a drug is rapidly distributed following its administration, a one-compartment model will do an adequate job of accurately and adequately characterizing the plasma concentration versus time data
Used in pharmacokinetics when it is necessary to describe the plasma concentration versus time data adequately and accurately
Provides the desired plasma concentration and duration of action for an administered drug
Allows accurate estimates of selected fundamental pharmacokinetics parameters like the apparent volume of drug distribution, the elimination half life, and the elimination rate constant of a drug
Facts and definition
The body is conceived to be composed of mathematically interconnected compartments
The complexity of ADME makes it necessary to sometimes assume a simplified model
The most useful model in pharmacokinetics
Linear pharmacokinetics
Drug transfer in the body is possibly mediated by passive diffusion
Principle of passive diffusion and the relationship between the rate of transfer and the administered dose of a drug
There is a directly proportional relationship between the observed plasma concentration and the amount of drug eliminated in the urine and the administered dose of the drug
Sites of drug administration
Imporant features of extravascular routes of drug administration
Plasma concentration versus time (RL) following oral administration of an identical dose of a drug via identical dosage forms with different formulations
The entire administered dose of a drug may not always reach general circulation (i.e. incomplete absorption)
The onset of action is determined by factors such as formulation and type of dosage form, route of administration, physicochemical properties of drug, and other physiological variables
An absorption phase is present
Inhalation
Rectal
Subcutaneous
Intramuscular
Oral
Sublingual or buccal
Transdermal
Intravascular route
Routes
Intravenous
Intra-arterial
Plasma concentration versus time plot (RL) following the administration of a drug by an intravascular route
Important features of the intravascular route of drug administration
Adverse reactions are difficult to reverse or control
Accuracy in calculations and administration of drug dose is critical
The route is used more often in life-threatening situations
The entire administered dose is available to produce pharmacologic effects
There is immediate onset of action
There is no absorption phase
Review of ADME processes
Disposition
Defined as all processes that occur subsequent to the absorption of the drug
The components of disposition
When the distinction is undesirable or unclear, disposition is the term used
Distinction between elimination and distribution
Once a drug is in systemic circulation, it is distributed simultaneously to all tissues including the organ responsible for its elimination
Elimination
Methods
Excretion
Other organs
Mother's milk (for infants)
Drug may be consumed in sufficient quantity to affect the infant
Not a significant route of elimination for mother
Lungs
Occasionally may be important for the elimination of substances with a high vapor pressure (e.g. gaseous anesthetics, alcohol, etc.)
Principal organs
Kidney
Primary site for removal of a drug in a chemically unaltered or unchanged form (i.e. excretion) as well as for metabolites
Liver
Primary organ where drug metabolism occurs
The irreversible loss of a drug in a chemically unchanged or unaltered form
The irreversible loss of drug from the site of measurement
Metabolism
Usually, metabolites posses little or none of the activity of the parent drug (there are exceptions)
The process of conversion of one chemical species to another chemical species
Distribution
Rate and extent of drug distribution is determined by
Controlled and determined by the physicochemical properties and chemical structures of a drug molecule
The permeability of tissue membranes to the drug molecule
How well the tissues and/or organs are perfused with blood
The binding of a drug to plasma proteins and tissue components
The process of reversible transfer of drug to and from the site of measurement (usually blood or plasma)
Any drug that leaves the site of measurement and does not return has undergone elimination
Absorption
The process by which a drug proceeds from the site of administration to the site of measurement
Important Definitions and Descriptions
Amount of drug in the urine
Cumulative amount of drug in urine (Xu) against time
Used to obtain selected pharmacokinetic parameters of a drug as well as other useful information such as bioavailability
Therapeutic range
The plasma or serum concentration range within which the drug is likely to produce the therapeutic activity or effect
Termination of action
The time at which the drug concentration in the plasma falls below the minimum effective conentration (MEC)
Duration of action
The time span from the beginning of the onset of action to the termination of action
Onset of action
The time at which the administered drug reaches the therapetuic range and begins to produce the effect
Relationship between the administered dose and the amount of drug in the body
Extravascular route
F * Dose = FX₀ = (AUC)₀∞ * KV
The amount of drug that reaches general circulation is the product of the bioavailability fraction (F) and the dose administered
Intravenous solution
Dose = X₀ = (AUC)₀∞ * KV
V = (or Vd) drug's volume of distribution
The apparent volume into which a given mass of drug would need to be diluted in order to give the observed concentration
K = first-order elimination rate constant
(AUC)₀∞ = area under curve of plasma drug concentration versus time (AUC) from time zero to time infinity
The amount of drug that reaches general circulation is the dose administered
Biopharmaceutics
The study of the factors that influence the bioavailability of a drug in humans and animals and the use of this information to optimize pharmacological and therapeutic activity of drug products
Factors
Physicochemical properties of drugs
Polymorphism
Partition coefficient
Size of distribution
Particle size
pKa
Method of manufacture
Wet granulation
Dry granulation
Inert excipients used in the formulation of a dosage form
Others!
Coloring agents
Disintegrating agents
Binding agents
Diluents
Chemical nature of a drug
Weak acid
Weak base
Pharmacokinetics
Applications
Clinical prediction
Using pharmacokinetic parameters to individualize the drug dosing regimen and thus provide he most effective drug therapy
Evaluation of drug interactions
Correlation of pharmacological responses with administered doses
Dosage adjustment of drugs in disease states, if and when necessary
Effects of physiological and pathological conditions on drug disposition and absorption
Bioavailability measurements
The study of kinetics of absorption, distribution, metabolism, and excretion (ADME) of drugs and their corresponding pharmacological, therapeutic, and toxic responses in humans and animals