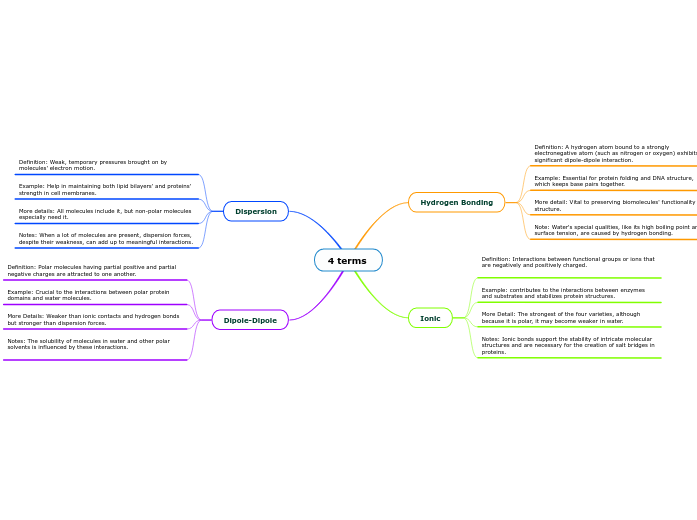
4 terms
Make your reading plan for this year!
Make sure that you will set realistic goals.
There are several ways to make a reading plan, by month is the simplest. You can pair each month with a genre, plan out what books you want to read in the most busiest month of the year and what to read on vacation!
Dipole-Dipole
Notes: The solubility of molecules in water and other polar solvents is influenced by these interactions.
More Details: Weaker than ionic contacts and hydrogen bonds but stronger than dispersion forces.
Example: Crucial to the interactions between polar protein domains and water molecules.
Definition: Polar molecules having partial positive and partial negative charges are attracted to one another.
Dispersion
Notes: When a lot of molecules are present, dispersion forces, despite their weakness, can add up to meaningful interactions.
More details: All molecules include it, but non-polar molecules especially need it.
Example: Help in maintaining both lipid bilayers' and proteins' strength in cell membranes.
Definition: Weak, temporary pressures brought on by molecules' electron motion.
Ionic
Notes: Ionic bonds support the stability of intricate molecular structures and are necessary for the creation of salt bridges in proteins.
More Detail: The strongest of the four varieties, although because it is polar, it may become weaker in water.
Example: contributes to the interactions between enzymes and substrates and stabilizes protein structures.
Definition: Interactions between functional groups or ions that are negatively and positively charged.
Hydrogen Bonding
Set your reading goal for the month!
You can even choose a preferred hour of the day or days of the week when you want to read.
Note: Water's special qualities, like its high boiling point and surface tension, are caused by hydrogen bonding.
More detail: Vital to preserving biomolecules' functionality and structure.
Example: Essential for protein folding and DNA structure, which keeps base pairs together.
Definition: A hydrogen atom bound to a strongly electronegative atom (such as nitrogen or oxygen) exhibits a significant dipole-dipole interaction.