por Samia Bakr hace 4 años
375
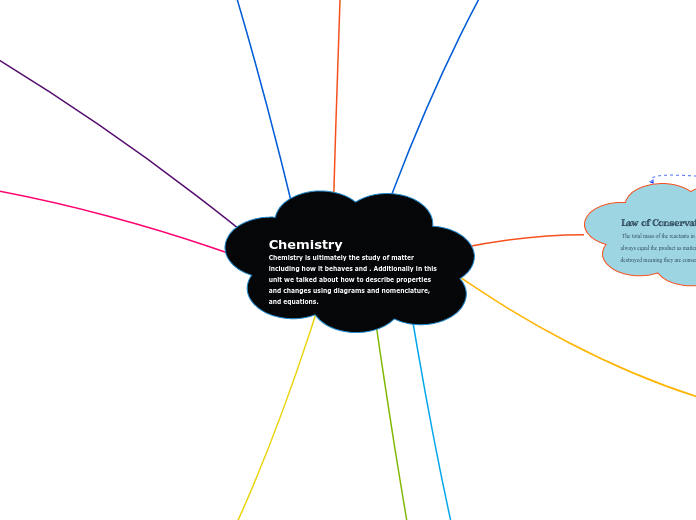
Acid-Base Equilibrium
The text discusses the critical aspects of acid-base equilibrium, focusing on the behavior and interaction of acids and bases in aqueous solutions. It highlights the process of titration, where the concentration of acids and bases can be determined using specific formulas.