por Kennedy Byars hace 4 años
262
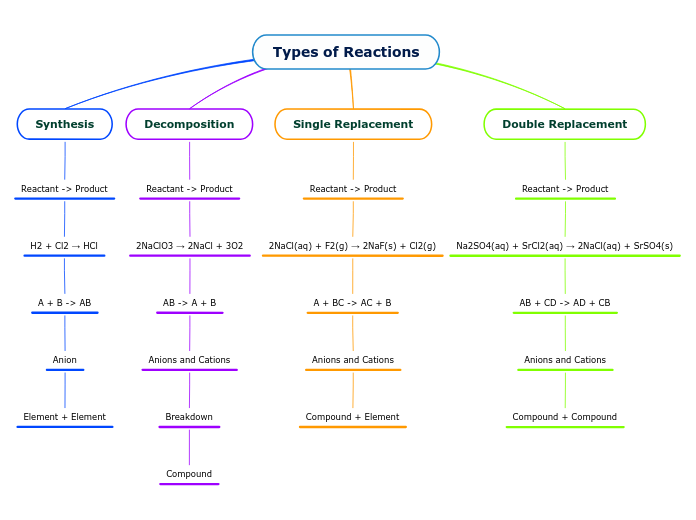
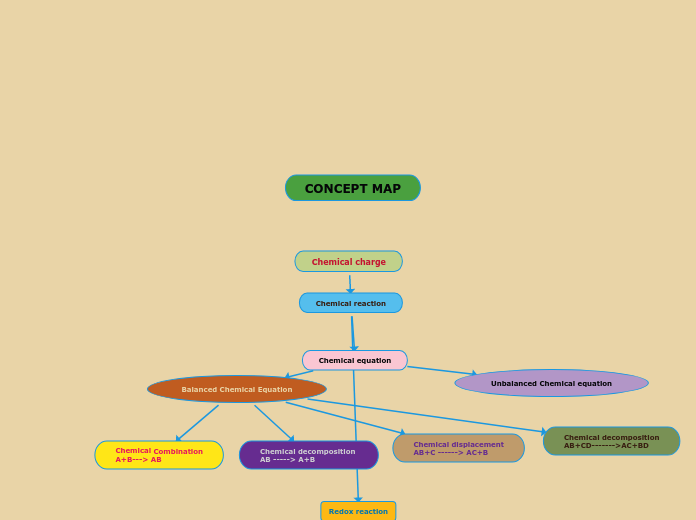
Types of Reactions
Chemical reactions can be categorized into several types based on the nature of the reactants and the products they form. Single replacement reactions involve one element displacing another in a compound, resulting in a new element and a new compound.