jonka Angel Torres 6 vuotta sitten
472
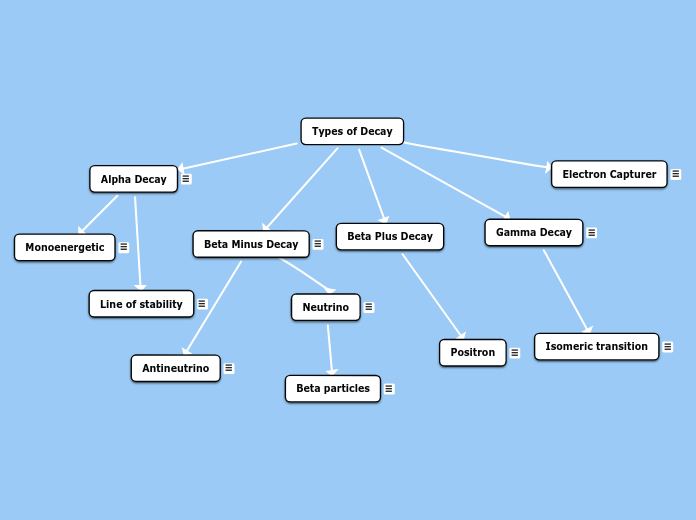
Atomic Nucleus
jonka Angel Torres 6 vuotta sitten
472

Lisää tämän kaltaisia
Its nucleus is proton rich and it has enough energy to convert a proton to a neutron, thus ejecting a positive electron or positron. Positrons loses energy over a short distance, then it interacts with a negative electron from another atom. In positron decay the atomic number increases by 1.
Gamma emissions represents a nucleus that released energy when excited. It is the process for releasing energy from a metastable nucleus. Gamma is emmited when there are more than 100KeV of excess energy in the excited nucleus.
Isomeric Transition refers to the release of energy such as gamma rays from a metastable state. The nucleus goes from higher energy level to a lower energy level through emission of electromagnetic radiation.
It has a neutron rich nucleus that becomes stable by converting neutrons into protons. The mass number of the nucleus remains the same, however the atomic number increases by 1 (a different element is created). The formation of another element is refer to as transmutation. The beta minus particle is ejected during this process.
Just like antineutrinos, neutrinos have no electric charge and both have a mass of zero. They also travel the speed of light and are undetectable.
The reason why Beta particles have different energies is due to the unequal sharing of kinectic energy between the electron and the neutrino. Sometimes electrons recieve more energy than neutrinos or vice versa.
During transmutation in beta minus decay, antineutrino is formed carrying part of the energy of this reaction.
Occurs within the heavy nucleus where four nucleons can achieve an energy greater than the nuclear binding energy to leave the nucleus. It is also the least penetrating type of radiation.
Monoenergetic refers to the particles having a single energy
Stability is mainly achieved when there is an even number of protons and neutrons. For example, alpha particles are composed of two protons and two neutrons hence been stable.
When orbital electrons are captured, they combine with a proton to form a neutron. Most of the electrons involved are usually from the K or L shell. Outer shell electrons move inward to fill the space created.