jonka Angel Peng 4 vuotta sitten
306
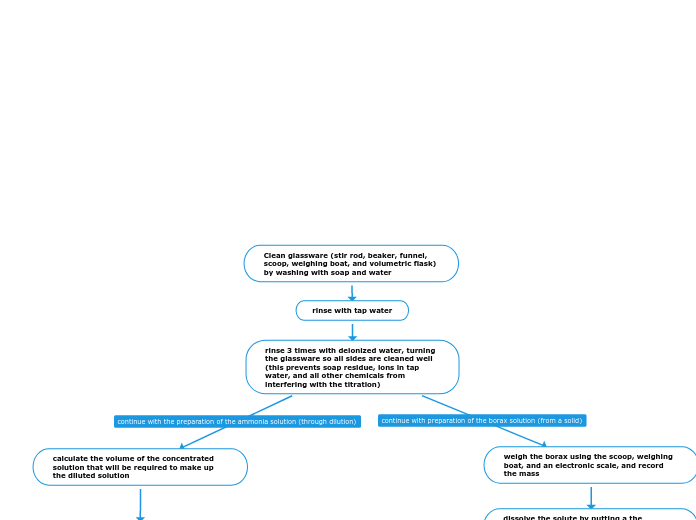
Clean glassware (stir rod, beaker, funnel, scoop, weighing boat, and volumetric flask) by washing with soap and water
jonka Angel Peng 4 vuotta sitten
306

Lisää tämän kaltaisia
prime your containers by pouring a small amount of the concentrated solution into a beaker, and pour this into the graduated cylinder and rinse both out into the waste beaker (priming ensures the only liquid in the containers is the concentrated solution)
measure the amount of concentration solution
record the volume in the graduated cylinder
transfer that volume into the volumetric flask by using and lifting the funnel
rinse the graduated cylinder 3 times with deionized water and pour it into the flask
continue to fill the volumetric flask to the fill line
pour it into a beaker and then into the graduated cylinder
dissolve the solute by putting a the minimum amount of deionized water needed to dissolve the solute in a beaker
pour the solute in the beaker, and rinse the weighing boat 3 times to ensure all solute weighed is put into the solution
stir the solution with a glass rod to further dissolve the solute
decant the solution slowly into the volumetric flask to ensure that no undissolved solute is put into the flask
place the stir rod on top of the beaker to allow a stream and to prevent splashing (hooking two fingers to keep it in place), and use your other hand to lift the funnel (to prevent the solution from building up in the funnel)
repeat procedure to dissolve all of the remaining solute by adding more deionized water, dissolving, and decanting
after all the solute is dissolved rinse the beaker 3 times and pour into the flask
fill the remaining volume of the flask with deionized water (slowing down when approaching the fill line to prevent going over and adding drop by drop using the stir rod if needed)
the flask has been filled when the bottom of the meniscus is touching the fill line
properly cleaning all materials
prime the burette with the borax (primary standard) titrant by pouring (with the glass rod and funnel technique) in a small amount and opening the stopcock to allow the solution into a waste beaker.
fill the burette with the borax solution to the highest fill line and record the starting volume
place a pipette into the beaker with the HCl and take an aliquot by squeezing and holding a bulb at the end of the pipette creating a seal, and also place your thumb over the end whenever the bulb is removed
release the aliquot into the clean flask by slowly releasing your thumb
prime the pipette by placing a small amount of HCl in a beaker and then rinse it out in a waste beaker
place a small amount of HCl back in the beaker and discard again into the waste beaker
take an aliquot using the same technique
add the appropriate amount of methyl orange indicator into the sample flask (because it changes color at neutralization which would indicate the endpoint of the titration) and swirl the flask (we use the endpoint since it is close to the equivalence point)
record the starting volume of the burette and leave your final digit as an estimate
do a quick first trial to estimate where the end point is
one hand remains on the stopcock (to stop the slow of the solution and use the most exact amount of solution needed)
the other hand is on the neck of the flask of the sample solution, swirling the flask contiuously
keep titrating until you notice a complete color change because this is the endpoint (this will be an intermediate color between the two colors of the indicator)
subtract the initial volume from the final volume to determine how much of the titrant was added into the flask in order to reach a point where the reactant was totally consumed
rinse and clean your materials and repeat the process but more precisely by slowing the flow of titrant when you approach the estimated endpoint
start the titration process again but this time repeat replacing the borax for HCl and the HCl for ammonia