jonka Siti Zahrah 6 vuotta sitten
2345
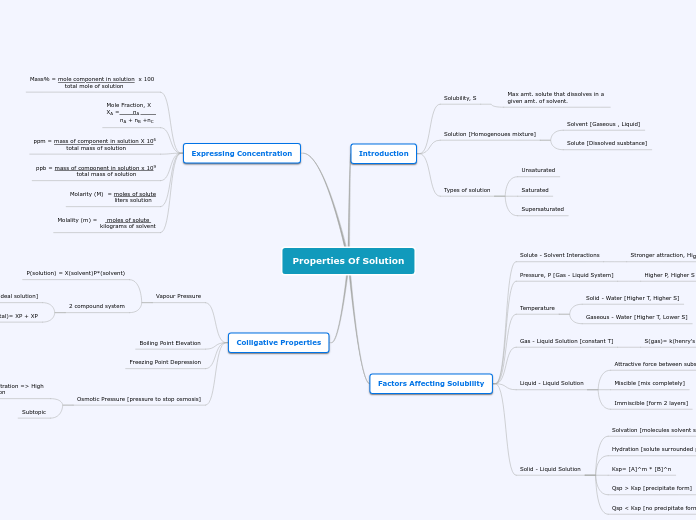
Properties Of Solution
jonka Siti Zahrah 6 vuotta sitten
2345

Lisää tämän kaltaisia
P(total)= XP + XP
Raoult's Law [ideal solution]