jonka Bains Mahabir 2 vuotta sitten
379
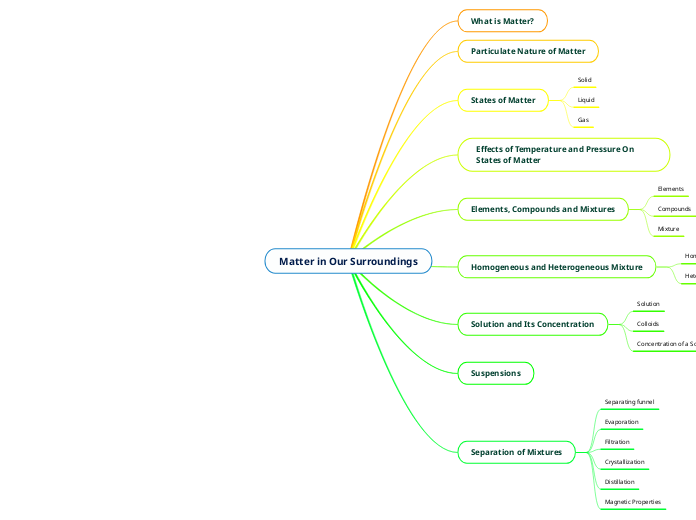
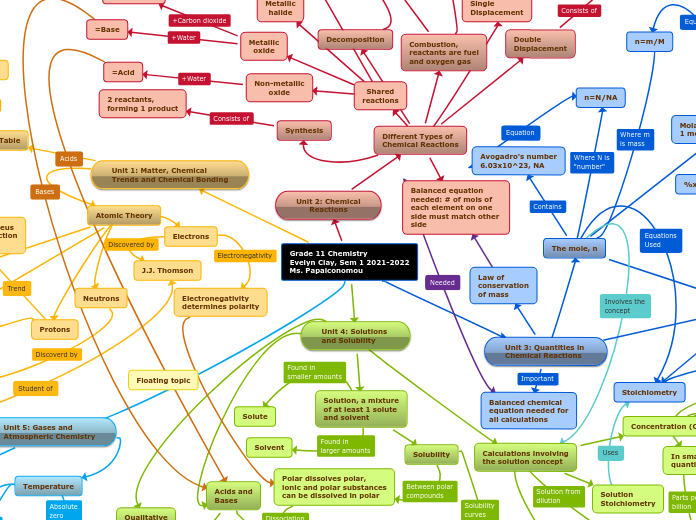
SCH3U Final Summative Concept Map
The provided text outlines various competencies and tasks related to chemistry, particularly in the areas of chemical bonding and compound identification. It emphasizes the ability to convert concentration units, predict reaction outcomes using solubility charts, and understand the properties of ionic and covalent compounds.