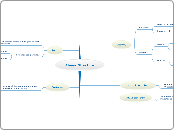
Atomic Structure
Atoms
Electron
Mass: - 1/1840 A.M.U
Charge: - -1
Nucleus
Proton
Charge: - +1
Mass: - 1 A.M.U
Neutron
Charge: - 0
Mass: - 1 A.M.U.
Ions
Are formed when atoms gain or lose electrons
Are charged particles
Positive: - Cations
Lost electrons
Negative: - Anions
Gained electrons
Isotopes
Atoms of the same element with different atomic mass
Due to differing numbers of neutrons
Atomic number
The number of protons or electrons in an atom
Mass number
The number of protons + the number of neutrons