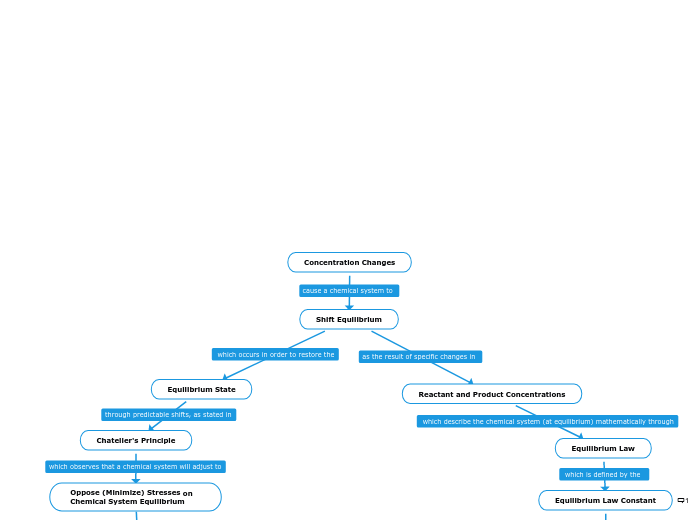
Concentration Changes
Shift Equilibrium
Equilibrium State
Chatelier's Principle
Oppose (Minimize) Stresses on Chemical System Equilibrium
Reactant and Product Concentrations
Equilibrium Law
Equilibrium Law Constant
Position of the Equilibrium
Change Concentration of Entities in Equilibrium
Increase in [Products]/ Decrease in [Reactants]
Reactants < Products
Reverse Reaction (Reduce [Product])
Reactants Form as Products Concentration Decrease
Reactant Production ↽
[Reactant] Decrease
Increase in Reactants/Decrease in Products Concentration
Reactants > Products
Forward Reaction (Reduce [Reactant])
Products Concentration Increase as Reactants Concentration Decrease
Collision Theory
Increase Particle Collision Frequency (Probability of Successful Collisions)
Formation (Concentration) of Products
[Reactants] must be Used Up
Decomposition into Reactants
[Products] must be Used Up
Equilibrium Graphs
Product Production ⇀
[Product] Decrease