Science Grade 10
Biology
Microscopes 🔬
Cells
The Cell Cycle
Mitosis
Prophase
First part of mitosis where the nuclear membran dissolves and disappears
Metaphase
The chromosomes become complletely condensed
Anaphase
The sister chromatids are pulled a part by the spindle fibers.
Telophase
Nuclear membrane reform to form new nuclei
Cancer
Stem Cells
Embryonic cells
Specialised Cells
Animal specialsed cells 🐻
Muscle Cells 💪
Contracts allowing bones to move.
Red Blood Cells 🔴
Carries oxygen through the blood
White Blood Cells ⚪
A cell that protects you from diseases.
Plant Specialsed Cells 🌱
Xlyem Cells
Responsible for transporting
water and dissolved minerals
Phloem
Responsible for
transporting sugar
Tissues
Animal tissues 🐻
Epithelial
Lines the outer surface of internal organs
Connective
Strengthens, supports and protects other tissues.
Muscle 💪
Moves the body or organ by contracting
Vascular
Epidermal
Ground
Meristematic
Hierarchy of organization
Interdependent organs
Interdependent organs work together to maintain body functions
Heart and Lungs
Heart pumps blood, lung help for circulation.
Brain and Muscles
Brain sends signals to muscles for movement and coordination
Kidneys and Bladder
Kidneys filter waste, bladder stores urine for excretion.
Liver and Digestive system
Liver processes nutrients from food for digestion and absorption.
Pancreas and small intestine
Pancreas secretes enzymes to aid digestion in small intestine.
Eye and brain
Eyes collect visual information, brain processes and interprets it.
Skin and circulatory system
Skin regulates temperature, circulatory system delivers nutrients to skin.
Endocrine and
reproductive system
Hormones from endocrine glands regulate reproduction and development.
Lungs and diaphragm
Diaphragm controls lung expansion for breathing and oxygen intake.
Skeletal and muscular system
Muscles move bones for body movement and posture.
Medical Imaging Technologies
Medical Resonance Imaging
Uses magnetic fields and radio waves to create detailed body images.
CT-Scan
Combines X-ray images for detailed cross-sectional views of the body.
Ultrasound
Uses sound waves to produce images of soft tissues and organs.
PET-Scan
Detects radioactive tracers to observe metabolic activity in tissues.
X-Rays
Uses radiation to capture images of bones and organs.
Plant Cells 🌱
Cell Walls
Provides structural strength
Chloroplasts
Produce energy from photosynthesis
Animal Cells 🐻
Mitochondria 🦠
Power house of the cell
Magnifys objects that are to small to be seen with the naked eye
Physics⚡️
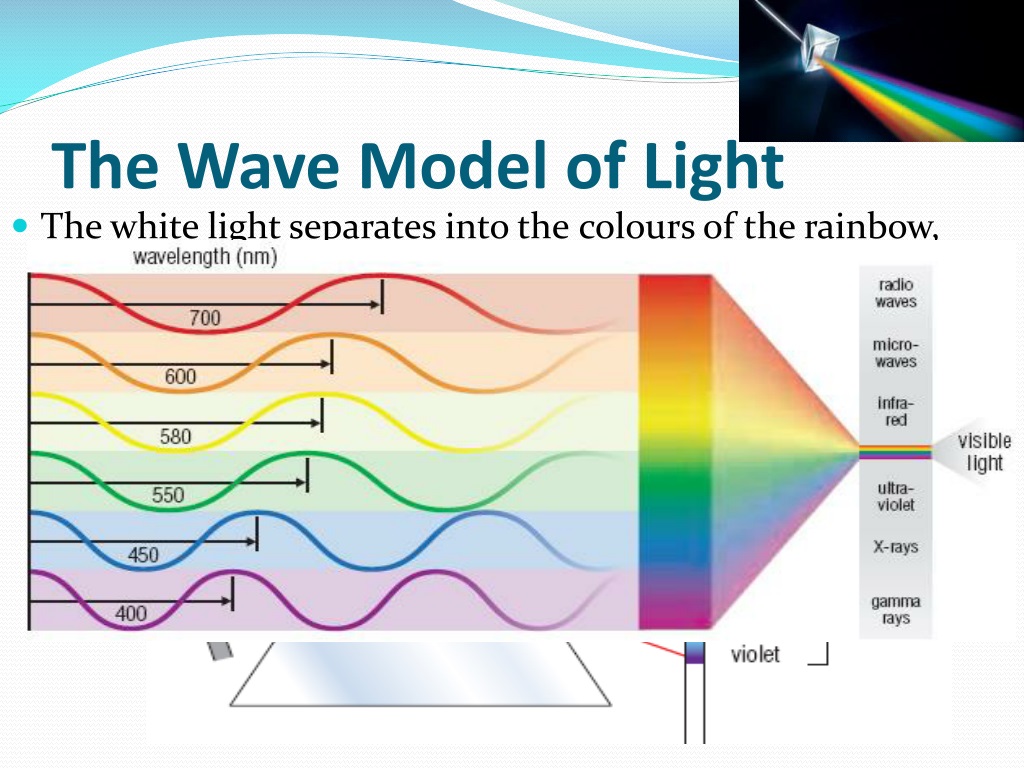
The Wave Model of Light
-When light passes through a prism, it separates into the colours of the rainbow
Chemistry🧪
Matter
States of matter
Liquid
Solid
Gas
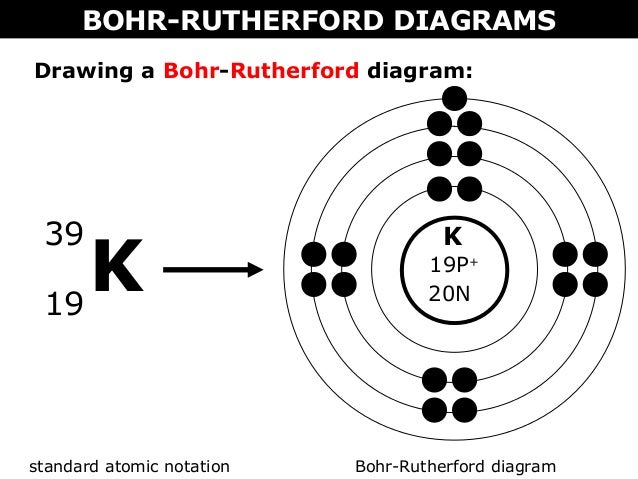
Atomic Structure
Periodic Table
Bohr Diagrams
Chemical Reactions
Synthesis
A + B →AB
Decompostion
AB → A + B
Single Displacement
A + BC → AC + B
Double Displacement
AB + CD → AD + CB
Combustion
When all substances in a compound are combined with oxygen
Examples
Color Change
Gas Production
Tempature Change
Ionic Compounds & Naming

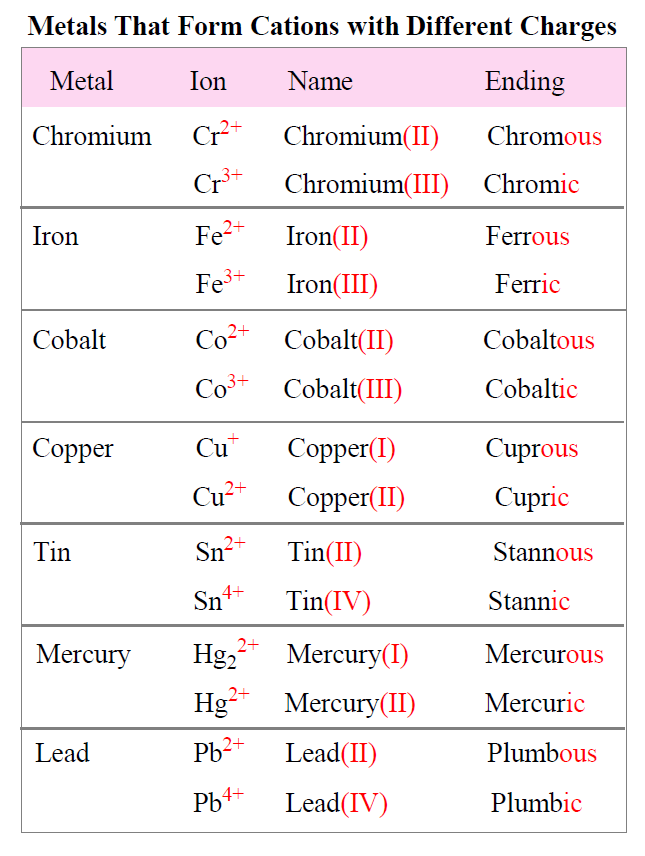
Transition Metals
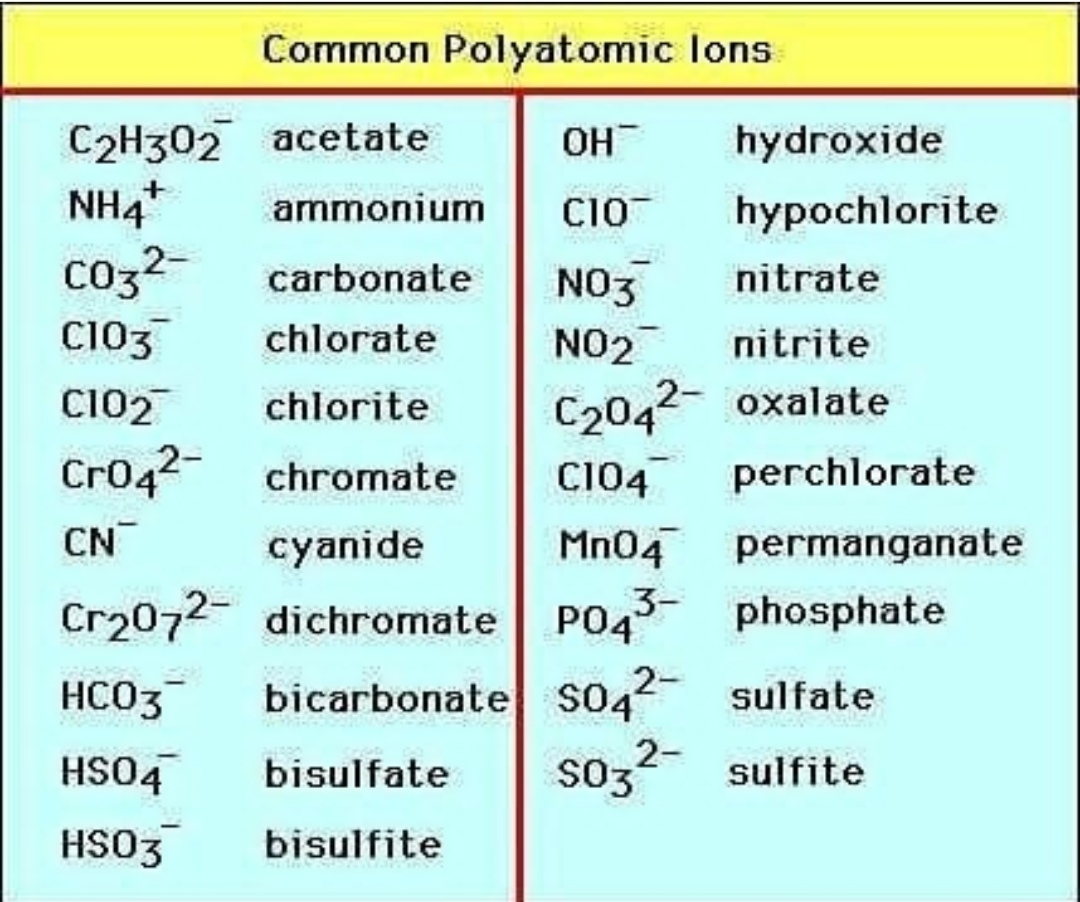
Polyatomic Ions
Molecular Compounds & Naming
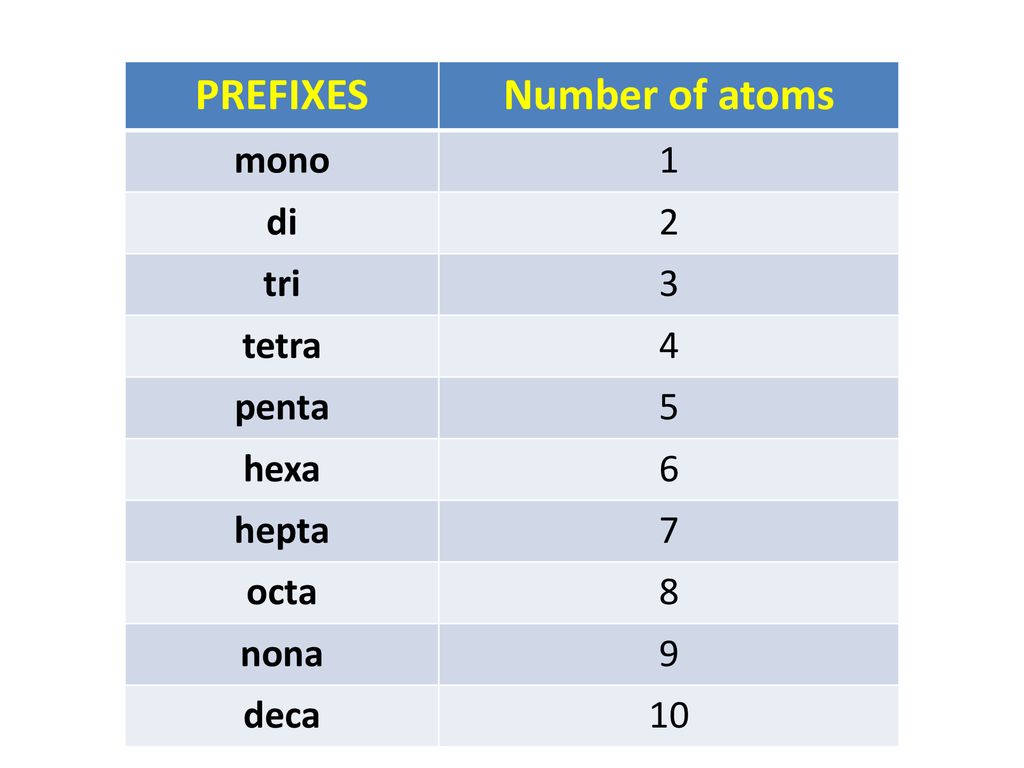
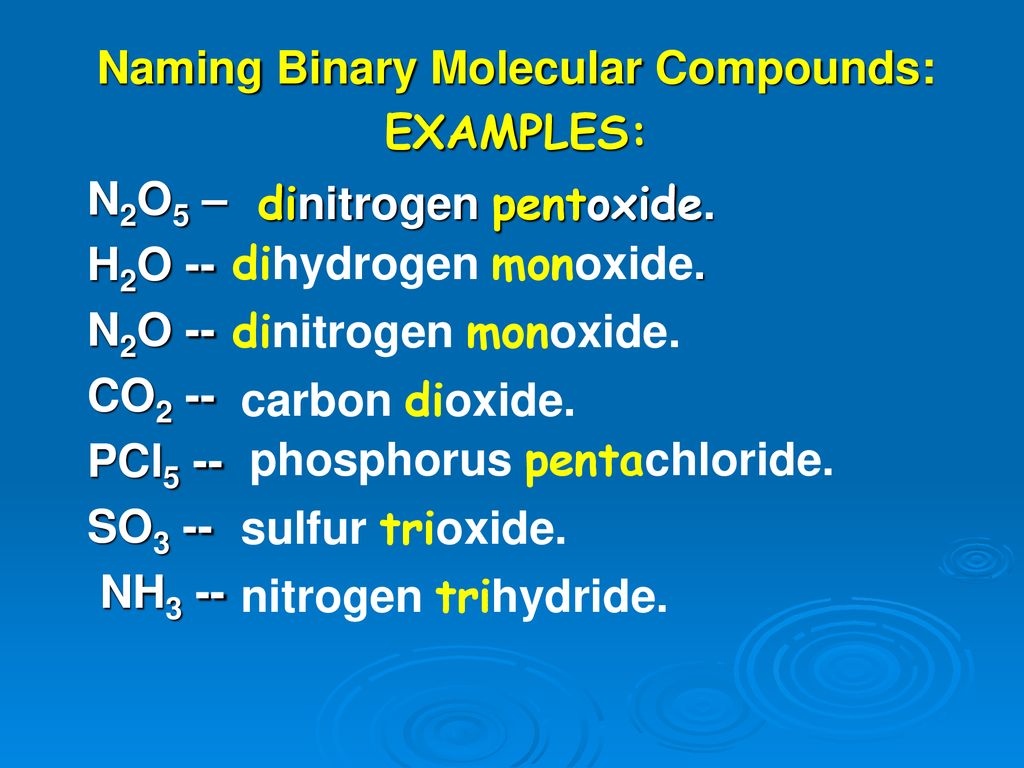
Counting Atoms
Law of Conservation Mass
Matter is neither created nor destroyed in a chemical reaction.
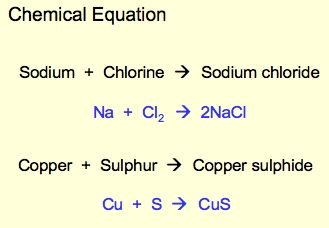
Word equations
Just like chemical equations only the elements in word form
Acids & Bases
Acids
A substance that produces hydrogen ions when dissolved in water
Sour such as lemons, vinegar
Like water
Good conductors of electricity
Non-metal oxides
Molecular compounds
Usually begins with "H"
Acids
A substance that produces hydroxide ions when dissolved in water
Bitter like tonic water
Slippery like soap
Good conductors of electricity
Metal oxides
ionic compounds
Usually ends with "OH" or "CO3"
Rules of Counting Atoms
1. The symbol of an element represents one atom of that element
NaCl= 2 atoms - Sodium-1
Chlorine-1
2. A subscript is a number written at the lower right corner after the symbol of an element. If there is more than one atom of the element, then a subscript is used to indicate the number of atoms of that element
H2SO4=7 atoms - Hydrogen-2
Sulfur-1
Oxygen-4
3. A subscript outside a bracket multiplies all the elements inside the brackets
Mg3(PO4)2=13 atoms -Magnesium-3
Phosphorus-2
Oxygen-8
4. A coefficient is a number written in front of a chemical symbol and indicates the number of atoms of that element. A coefficient can also be written in front of a chemical formula to indicate the number of molecules of that compound
3 CO2=9 atoms-Carbon-1x3=3
Oxygen-2x3=6
Neutralization
Acids + base→Salt + Water
Groups
Alkali Metals
Alkaline Earth Metals
Halogens
Noble Gases
Element Properties
Metals
Nonmetals
Metaloids
Subatomic Particles
Protons
A subatomic particle with a positive charge
Electrons
A subatomic particle with a negative charge
Neutrons
A subatomic particle with a neutral charge
Waves
Properties of waves
Wavelength
The distance from one wave to the next
Symbol for wavelength is the Greek letter Lambda λ
Amplitude
The wave's height or depth from the rest position
Frequency
The rate of repetition of a wave
The Electromagnetic Spectrum
Types of Light
Additive & Subtractive colour theory
Additive
White like is composed of different colours of light
Red
Orange
Yellow
Green
Blue
Violet
Subtractive
When light strikes an object, some waves are reflected and some are absorbed
Coloured matter absorbs different wavelength of light
The absorbed colours "subtracted" from the reflect light
Ray Model of Light
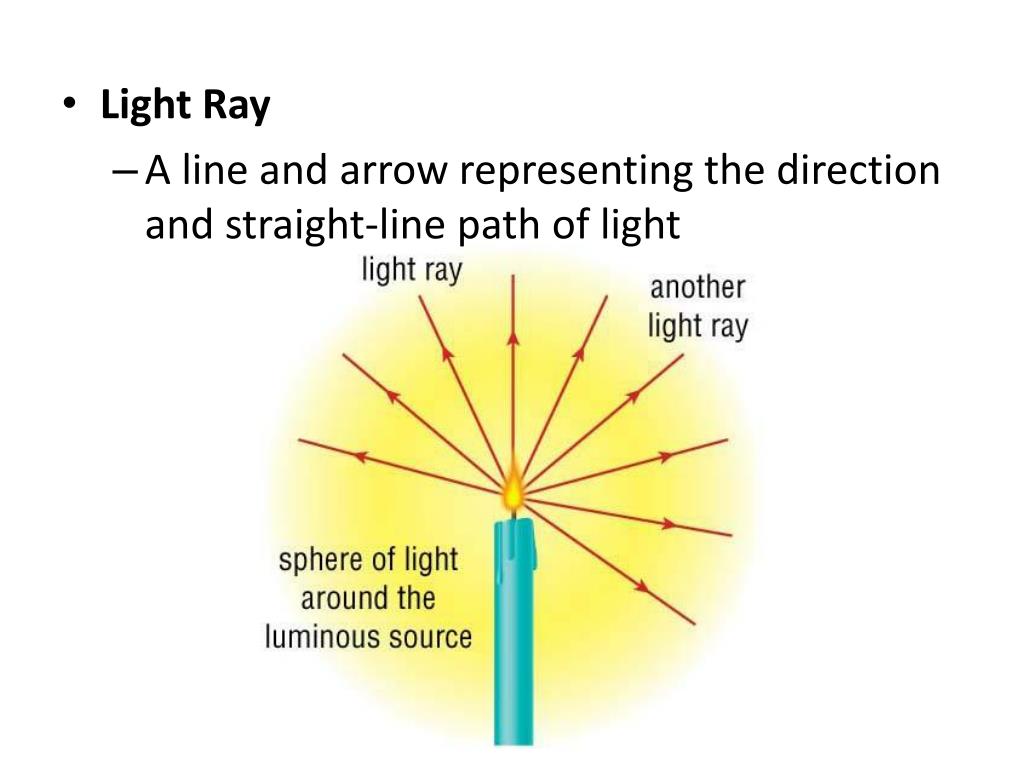
This diagram shows how light rays travel in a straight line away from a light source in every direction
Light and matter interaction
Absoption
Reflection
Light Rays interact in specific ways
Transparent Object
Light rays are transmitted
Translucent Object
Light rays are transmitted and reflected
Opaque Object
Light rays are reflected
Shadows
Dark areas where an object blocks direct light rays from a light source
Object near to the source = bigger shadow
Object far to the source = smaller shadow
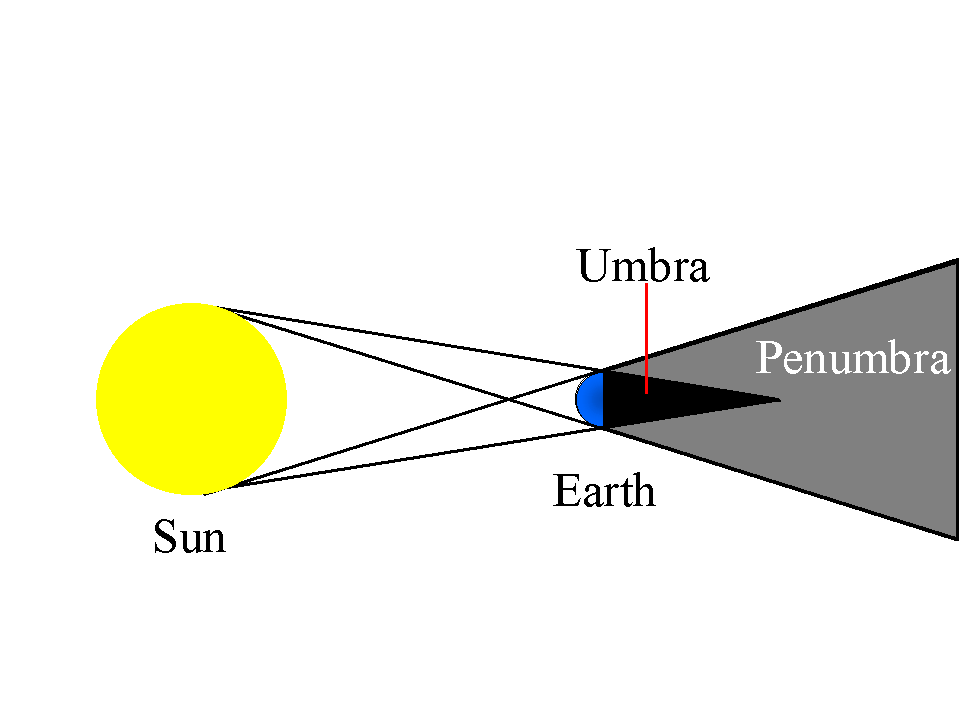
Umbra
Dark areas where all light rays from a source no matter how small or far are blocked
Penumbra
The partial shadow part where light rays from a non point source are party blocked
Transmission
Reflection & Refraction
Laws of reflection
Angle of Incidence = Angle of Reflection
:max_bytes(150000):strip_icc()/ReflectionLaw-5946c6dd5f9b58d58a2f2efc.png)
Plane mirrors
Virtual Images
Reflection
Concave mirrors
Real or Virtual images
Focus point
Convex mirrors
Virtual, smaller Images
Divergence rays
Refraction
The bending or change in direction of light when it travels from one substance to another
Index of Refraction
Light moves from a faster substance to a slower substance, the light will bend towards the normal and if the light moves from a slower substance to a faster substance, the light will bend away from the normal
Types of reflections
Specular Reflection
All rays are parallel

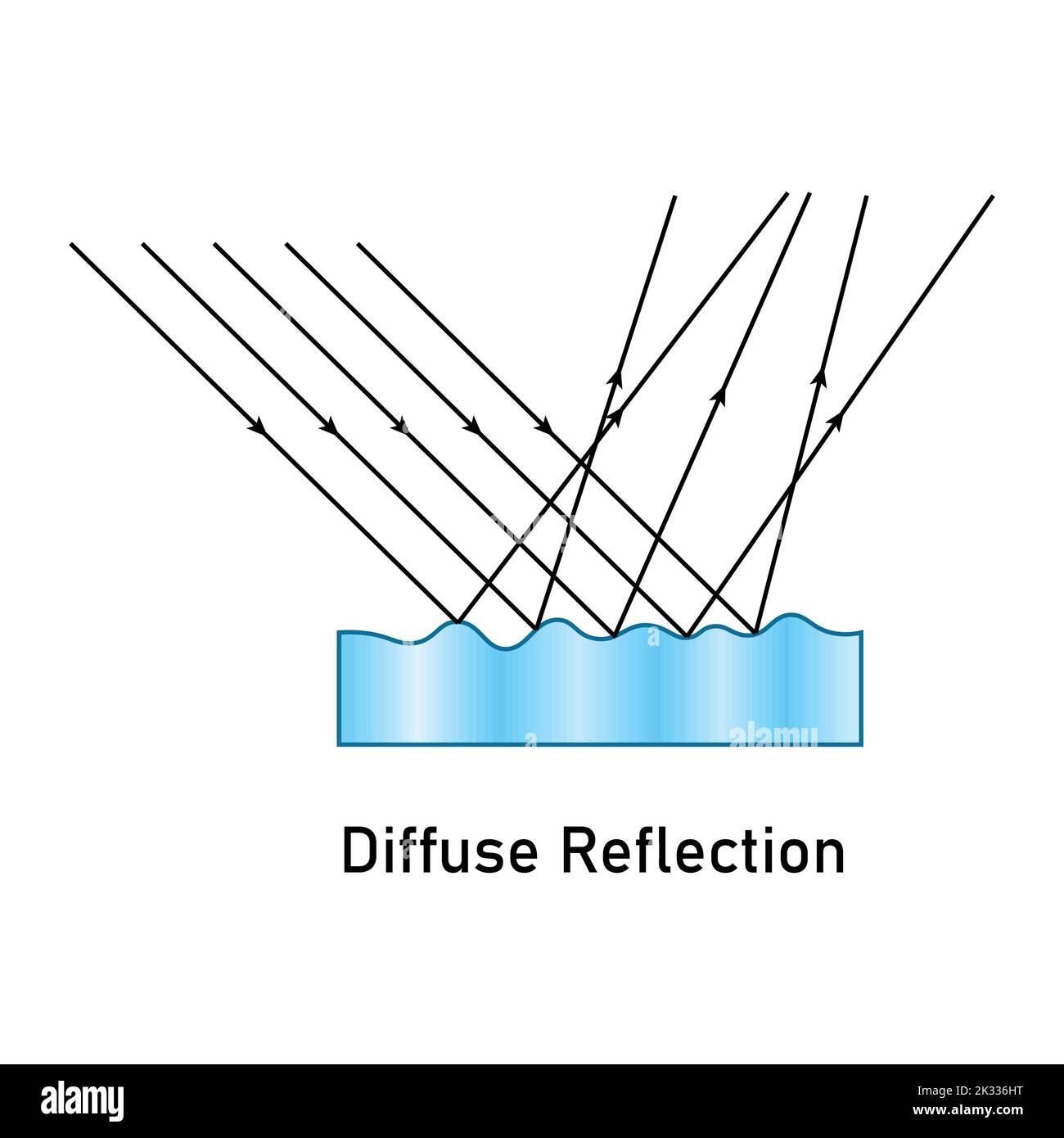
Diffuse Reflection
All rays are rays are moving in different directions
Concave and Convex lenses
In concave lenses, light converges at the focal point to create virtual images
Virtual images
Light rays only appear to come to that point
In Convex lenses, light converges at the focal point to create real images
Real images
Light rays actually meet at that point
SALT
Size
Mirrors/lenses
Attitude
Location
Type of image
Technical application of lenses and mirrors
Cameras, glasses, telescopes
The human eye
Convex lenses
Radio Waves
Radio, TV, Cell phones
Microwaves
Heat food, Radar, mapp the Earth and space objects
Infrared Waves
Felt as heat, alarms, NVG
Ultraviolet Rays
Produced by Sun and Stars, burn skin and increase risk of cancer, disinfect water
X-Rays
Penetrate human tissue for medical imaging, luggage scanning
Gamma Rays
Penetrate human tissues to kill cancer cells
White light
Diagram
Describes image formed by
Cell
Tissues
Organ system
Organsim
Humans
Endocrine system
Regulates metabolism, growth, and mood through hormones.
Reproduction system
facilitates reproduction, supports fetal development.
Integumentary system
Protects body, controls temperature
Skeletal system
Provides structure, protects organs, stores minerals, allows movement.
Muscular system
Allows movement, maintains posture, generates body heat.
Nervous system
Controls body activities, processes sensory information, plans responses
Cardiovascular system
Circulates blood, nutrients, oxygen, and removes waste
Lymphatic system
Defends against infection, maintains fluid balance, absorbs fats.
Respiratory system
Exchanges oxygen and carbon dioxide, maintains blood pH balance.
Digestive system
Breaks down food, absorbs nutrients, eliminates waste.
Urinary system
Eliminates waste, regulates fluid and electrolyte balance, controls pH.