Sewage/ Wastewater Treatment
By: Akshaya Madduru
TECHNOLOGY
THE IMPACT
What is the technological impact of sewage treatment?
THE PROS
The development of MBBR (1980) :
Mixed Bio Reactor technology
THE CONS
Cost

Time
SOCIETY
THE IMPACT
What is the societal impact of sewage treatment plants?
THE PROS
Larger supply of clean water
WORK CITED
jain, Neha. “What Are the Benefits and Drawbacks of Using MBBR Technology on Sewage Treatment Plant? Pages 1 - 3 - Flip PDF Download: FlipHTML5: Neha Jain.” FlipHTML5, Neha Jain, 24 July 2020, fliphtml5.com/uqwis/btop/basic.
BarcelonaTech. “Environmental Benefits.” ECUVal, 2017, www.ecuval.eu/enviromental-benefits.
Middleton, Judith. Environmental, Economic and Social Impacts of the Use of Sewage Sludge on Land Final Report Part I: Overview Report. Milieu Ltd, WRc and RPA for the European Commission, 2008.
Person. Chemical Treatment of Wastewater Process, Thomas, www.thomasnet.com/articles/chemicals/wastewater-chemical-treatment/#:~:text=Chemicals%20are%20used%20during%20wastewater,to%20achieve%20various%20water%20standards.
Tanks, Carlow. “The Benefits of a Wastewater Treatment System: Carlow Tanks.” Carlow Concrete Tanks, 24 July 2019, www.carlowtanks.ie/the-benefits-of-a-modern-wastewater-treatment-system/.
Technology, International Environmental. “How Does Sewage Affect the Environment?” Envirotech Online, 2016, www.envirotech-online.com/news/water-wastewater/9/breaking-news/how-does-sewage-affect-the-environment/40472.
vitz, ed. “Waste Water Treatment.” Chemistry LibreTexts, Libretexts, 18 Aug. 2020, chem.libretexts.org/Bookshelves/Ancillary_Materials/Exemplars_and_Case_Studies/Exemplars/Environmental_and_Green_chemistry/Waste_Water_Treatment.
Wilson, Sara. “Http://Legacycontent.halifax.ca/Harboursol/Documents/gpi_report_001.Pd.” Legacy Content, 2000, legacycontent.halifax.ca/harboursol/documents/gpi_report_001.pdf.
Introduction
WHAT IS SEWAGE TREATMENT?
HOW DOES IT WORK/
TREAT WATER?
Modern water treatment technology
WHAT ARE THE EQUATIONS:
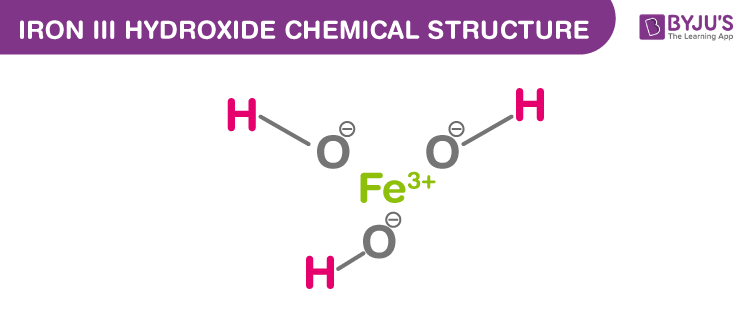
Net ionic equation:
3Fe2+(aq)+ MnO-4(aq) + 2H2O(l) → 3Fe3+(aq) + MnO2(S) + 4OH-(aq)
= Fe3+(aq) + 3OH-(aq) → Fe(OH)3(s)
= As you can see a precipitate is formed. and repetition of ions and elements on both sides did occur.

Net ionic equation:
the removal of Manganese(II):
Mn2+(aq) + Cl2(aq) → Mn4+ (aq) + 2Cl-(aq) : Mn4+(aq) + O2(g) → MnO2(s)
= Mn4+ was created in the product side, and makes insoluble precipitates, which caused problems. So to eliminate that, you have to add oxygen to balance out the Mn4+.
ENVIRONMENT
THE IMPACT
What is the environmental impact of sewage treatment?
THE PROS
THE CONS