par xavier wong Il y a 11 années
337
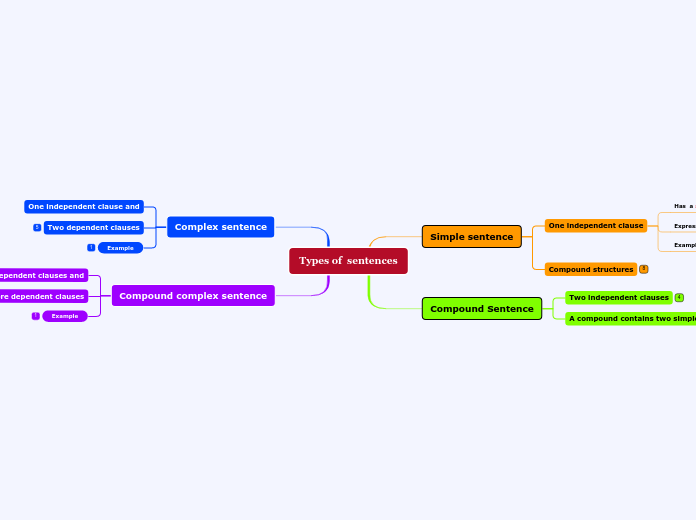
compound,mixture and element
The text discusses various types of substances and their characteristics. Compounds are formed from the combination of two or more elements in fixed proportions. Mixtures, on the other hand, consist of two or more substances that can be separated by physical methods and do not have fixed proportions.