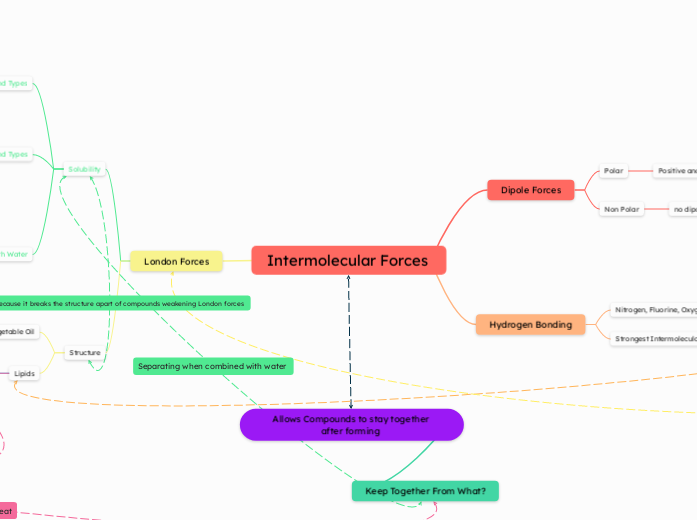
Allows Compounds to stay together after forming
Keep Together From What?
Intermolecular Forces
London Forces
Structure
Lipids
Saturated Fats
Makes Stronger London Forces
Which Makes Stronger Intermolecular Forces
Melting/Boiling Point
High
Will not melt at room tempature
Low
Will melt at room tempature
Unsaturated Fats
Makes Weaker London Forces due to structure being further apart
Which makes Weaker Intermolecular Forces
Vegetable Oil
Double Bond
Add Water Getting Rid of Double Bond
Structure Tightens
Hydrated Vegetable Oil
Solubility
With Water
Hydrophilic Compound's
Soluble with water
Polar and Ionic Bonds
Nonpolar bonds
Hydrophobic Compound's
Insoluble with water
Two Same Bond Types
Soluble With Each other
Two Different Bond Types
Insoluble With One Another
Hydrogen Bonding
Strongest Intermolecular Force
Nitrogen, Fluorine, Oxygen
Mustard Powder
Lipids, and Oils (Hydrophobic)
Reacts with the Oil
Creates London Forces
Carbohydrates, Proteins. (Hydrophilic)
Reacts with Vinegar. (Basically Water)
Creates Hydrogen Bonding
Stabilizes mixture of oil and vinegar
Creates Salad Dressing
Dipole Forces
Non Polar
no dipoles
Therefore no Dipole Forces
Polar
Positive and Negative Dipoles
Attract to surrounding dipoles of other molecules
Different mass and size
Weakens Dipole Forces Strength
Similar Mass and Size
Increases Dipole Forces Strength
Strength Also depends on polarity of the molecule