Intro to Chem .
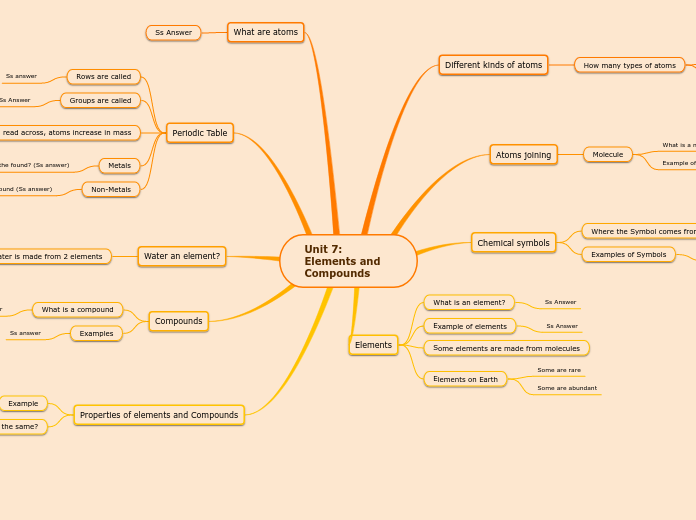
Periodic Table
groups into
nonmetals
noble gases
halogens
metals
alkaline earth metals
Alkaline earth metals have two valance electrons , have positive valance ,
alkali metals
Alkali metals are soft , highly reactive , have one valance electron , low melting points , & have positive valance .
Periods & groups .
is full of
Atoms
& have
nucleus
electrons
neutrons
protons
atomic mass
atomic number
Around Nucleus
Electrons ( Negative )
Inside Nucleus
Neutrons ( Neutral ) & Protons ( Positive ) .
isotope, atomic mass, atomic number, periodicity, periods, alkalai metals, alkaline earth metals, halogens, noble gases, ions, chemical reaction, reactants, products, reaction rate, kinetic theory