par Jonathan Mangar - Marvin Heights PS (1110) Il y a 4 années
980
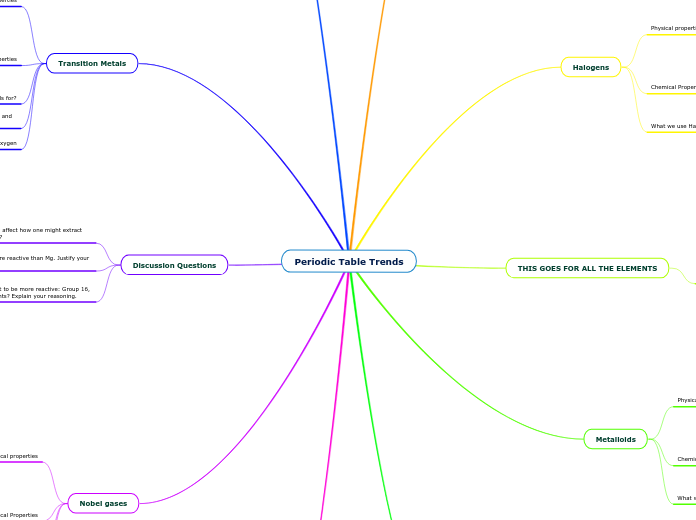
Periodic Table Trends
The alkaline earth metals, found in the periodic table, are characterized by low melting and boiling points, as well as low densities. These metals are key ingredients in fireworks due to their reactive nature.