par cesar romero Il y a 3 années
185
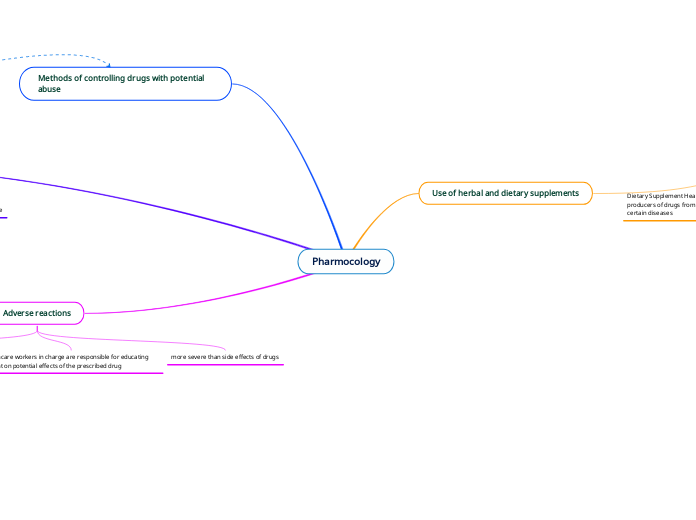
Pharmocology
The regulation of herbal and dietary supplements falls under the classification of foods, not drugs, meaning they are not overseen by the FDA. Consequently, these products can claim benefits for body structure and function but cannot assert they cure diseases due to the Dietary Supplement Health and Education Act.