References
- PT2448 CW Kinases and Phosphatases
- Hanks SK, Quinn AM, Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42-52. doi: 10.1126/science.3291115. PMID: 3291115
- Ardito F, Giuliani M, Perrone D, Troiano G and Lo Muzio L: The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy (Review). Int J Mol Med 40: 271-280, 2017
- Taylor SS, Radzio-Andzelm E, Hunter T. How do protein kinases discriminate between serine/threonine and tyrosine? Structural insights from the insulin receptor protein-tyrosine kinase. FASEB J. 1995 Oct;9(13):1255-66. doi: 10.1096/fasebj.9.13.7557015. PMID: 7557015.
- Knighton DR, Zheng JH, Ten Eyck LF, Ashford VA, Xuong NH, Taylor SS, Sowadski JM. Crystal structure of the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991 Jul 26;253(5018):407-14. doi: 10.1126/science.1862342. PMID: 1862342.
- PF6420 JJK PF6420 2020 3 Kinase Inhibitors
- Hubbard SR, Miller WT. Receptor tyrosine kinases: mechanisms of activation and signaling. Curr Opin Cell Biol. 2007 Apr;19(2):117-23. doi: 10.1016/j.ceb.2007.02.010. Epub 2007 Feb 16. PMID: 17306972; PMCID: PMC2536775.
- Siveen, K.S., Prabhu, K.S., Achkar, I.W. et al. Role of Non Receptor Tyrosine Kinases in Hematological Malignances and its Targeting by Natural Products. Mol Cancer 17, 31 (2018). https://doi.org/10.1186/s12943-018-0788-y
- Josso N, di Clemente N. Serine/threonine kinase receptors and ligands. Curr Opin Genet Dev. 1997 Jun;7(3):371-7. doi: 10.1016/s0959-437x(97)80151-7. PMID: 9229113.
- Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov. 2015 Feb;14(2):130-46. doi: 10.1038/nrd4504. PMID: 25633797; PMCID: PMC4480421.
- Bhullar, K.S., Lagarón, N.O., McGowan, E.M. et al. Kinase-targeted cancer therapies: progress, challenges and future directions. Mol Cancer 17, 48 (2018). https://doi.org/10.1186/s12943-018-0804-2
- Maurer G, Tarkowski B, Baccarini M. Raf kinases in cancer-roles and therapeutic opportunities. Oncogene. 2011;30:3477–88.
- Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554.
- Wu P, Nielsen TE, Clausen MH. FDA-approved small-molecule kinase inhibitors. Trends Pharmacol Sci. 2015 Jul;36(7):422-39. doi: 10.1016/j.tips.2015.04.005. Epub 2015 May 12. PMID: 25975227.
- Herceptin Summary of Product Characteristics (https://www.medicines.org.uk/emc/product/10041/smpc#INDICATIONS)
- PF6420 JJK Medicinal Chemistry of Anticancer agents: Breast Cancer treatment - Trastuzumab Emtansine
- Avastin Summary of Product Characteristics (https://www.medicines.org.uk/emc/medicine/15748/SPC/Avastin+25mg+ml+concentrate+for+solution+for+infusion/)
- Sutent Summary of Product Characteristics (https://www.medicines.org.uk/emc/product/227/smpc#gref)
- PF6420 JJK Irreversible Tyrosine Kinase Inhibitors
- Bowles DW, Diamond JR, Lam ET, Weekes CD, Astling DP, Anderson RT, et al. Phase I study of oral rigosertib (ON 01910.Na), a dual inhibitor of the PI3K and Plk1 pathways, in adult patients with advanced solid malignancies. Clin Cancer Res. 2014;20(6):1656-65.
- Gumireddy K, Reddy MVR, Cosenza SC, Nathan RB, Baker SJ, Papathi N, et al. ON01910, a non-ATP-competitive small molecule inhibitor of Plk1, is a potent anticancer agent. Cancer Cell. 2005;7(3):275-86.
- Jewer M, Findlay SD, Postovit L-M. Post-transcriptional regulation in cancer progression : Microenvironmental control of alternative splicing and translation. J Cell Commun Signal. 2012;6(4):233-48.
- Sawyers CL. Opportunities and challenges in the development of kinase inhibitor therapy for cancer. Genes Dev. 2003;17(24):2998-3010.
- E, Millward M, Ziman M. Advances in Personalized Targeted Treatment of Metastatic Melanoma and Non-Invasive Tumor Monitoring. Frontiers in oncology. 2013;3:54.
- Yin L, Morishige K, Takahashi T, Hashimoto K, Ogata S, Tsutsumi S, Takata K, Ohta T, Kawagoe J, Takahashi K, Kurachi H. Fasudil inhibits vascular endothelial growth factor-induced angiogenesis in vitro and in vivo. Mol Cancer Ther. 2007 May;6(5):1517-25. doi: 10.1158/1535-7163.MCT-06-0689. PMID: 17513600.
- Robert Roskoski Jr. Properties of FDA-approved small molecule protein kinase inhibitors.Pharmacological Research 144 (2019) 19–50
- https://clinicaltrials.gov/ct2/show/NCT04032171
- Anish Thomas, Arun Rajan, and Giuseppe Giaccone.Tyrosine Kinase Inhibitors in Lung Cancer.Hematol Oncol Clin North Am. 2012 June ; 26(3): 589–605.
- Ryohei Katayama, Christine M. Lovly, and Alice T. Shaw.Therapeutic Targeting of Anaplastic Lymphoma Kinase in Lung Cancer: A Paradigm for Precision Cancer Medicine.
- Drilon et al.Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three phase 1–2 trials.December 11, 2019. Lancet Oncol 2020; 21: 261–70
- Kei Ishii, Nao Morii, Hiroyasu Yamashiro.Pertuzumab in the treatment of HER2-positive breast cancer: an evidence-based review of its safety, efficacy, and place in therapy.Core Evidence 2019:14 51–70
- Hamid Maadi, Babak Nami , Junfeng Tong, Gina Li and Zhixiang Wang.The effects of trastuzumab on HER2-mediated cell signaling in CHO cells expressing human HER2. Maadi et al. BMC Cancer (2018) 18:238.
- José Baselga et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial.Lancet. 2012 February 18; 379(9816): 633–640.
- Jun-Cheng Xuhong , Xiao-Wei Qi , Yi Zhang, Jun Jiang.Mechanism, safety and efficacy of three tyrosine kinase inhibitors lapatinib, neratinib and pyrotinib in HER2-positive breast cancer.Am J Cancer Res 2019;9(10):2103-2119
- Melania Poratti, Giovanni Marzaro. Third-generation CDK inhibitors: A review on the synthesis and binding modes of Palbociclib, Ribociclib and Abemaciclib. European Journal of Medicinal Chemistry 172 (2019) 143e153
- Ingo Hartlapp, Christian Pallasch, GannaWeibert , Andrea Kemkers, Michael Hummel, Daniel Re. Depsipeptide induces cell death in Hodgkin lymphoma-derived cell lines.Leukemia Research 33 (2009) 929–936
- C. Owen md, N.L. Berinstein md, A. Christofides msc rd and L.H. Sehn md.Review of Bruton tyrosine kinase inhibitors for the treatment of relapsed or refractory mantle cell lymphoma.Current Oncology, Vol. 26, No. 2, April 2019.
- Maria Larrosa-Garcia and Maria R. Baer.FLT3 inhibitors in acute myeloid leukemia: Current status and future directions.Mol Cancer Ther. 2017 June ; 16(6): 991–1001
- Mingzhen Zhang, Hyunbum Jang and Ruth Nussinov. PI3K inhibitors: review and new strategies.Chem. Sci., 2020, 11, 5855
- Hélène Haguet Jonathan Douxfils Christian Chatelain, Carlos Graux, François Mullier, Jean-Michel Dogné.BCR-ABL Tyrosine Kinase Inhibitors: Which Mechanism(s) May Explain the Risk of Thrombosis?.TH Open 2018;2:e68–e88.
Protein Kinases
by:
Ciarán Connery
Emily Moloney
Ciarán Smiddy
Protein
Kinases
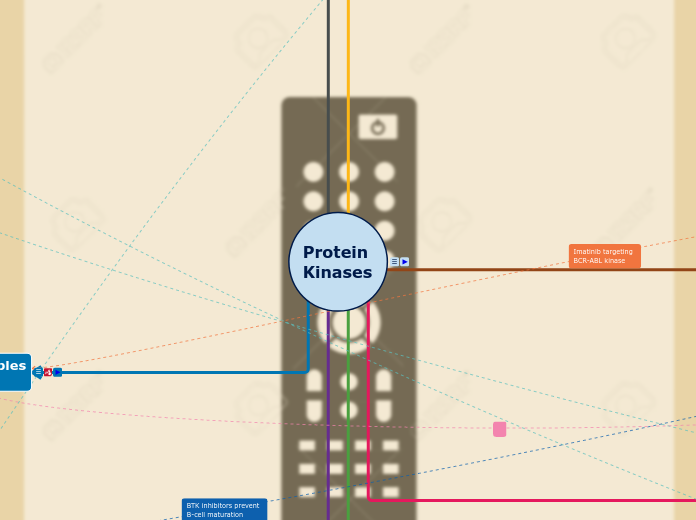
Protein kinases control the phosphorylation of proteins, which can be modified to orchestrate many cellular processes. Hence our design is based on a TV remote control, switching on and off the activities of certain cells[ e.g. growth, repair, death etc] and illustrating how protein kinases control the phosphorylation of proteins. Through the targeting of specific protein kinases implicated in disease states, it is possible to control and modify the disease.
PK inhibitor
Chemical features
Allosteric binding
Groups
These groups may be either hydrophilic or hydrophobic and are often involved in extending the half life of the drug in its interaction with the kinase. These groups don't have any affect on the specificity of the drug in question. 26
Hydrophobic groups
These groups also extends activity of the drug without ,usually, conferring additional specificity. A p loop group, which is usually hydrophobic may however, allow for better binding to Janus kinase so it is not always lacking the ability to allow greater specificity and a MEK kinase hydrophobic group which is usually made up of a 1-fluoro 3-iodo benzene ring. 26
Hydrophilic
Groups
These groups rarely are involved in the activity of the[ with the rare exception of certain allosteric binding sites needing hydrophilic groups.] For the most part, these groups aim to modify the hydrophilicity-lipophilicty profile of the drug allowing oral administration. 26
Cyclic Nitrogenated
Groups
Nitrogenated groups are involved in the adenine site which is essential for its activity as it aims to interact with the ATP binding site preventing Kinase activation. Groups that may be involved include Pyridine, isoquinoline, pyrazine,piperidine, imidazole but most commonly it is a pyrimidine group[ shown]
Other cyclic groups like a ribose like moiety may be added to a compound to extend its duration of action and half life but it should be noted that this is rarely ever used. 26
Disease State examples
and Treatment
It should be noted that Protein kinase inhibitors can treat numerous other conditions barring cancer including Glaucoma[ Netarsudil (AR11324)], Thrombocytopenia [Fostamatinib], used in transplants[Sirolimus], Ideopathic pulmonary Fibrosis[ Nintedanib], Rheaumatoid arthritis[ Tofactinib, Baricitinib] 26 and further development is being done on its use in the treatment of IBD, Asthma, Lupus and even a new protein kinase drug against ,Bruton kinase, Evobrutinib is in phase 2 trials to treat Multiple Sclerosis. 27
Lymphoma
Lymphoma is any form of blood related cancer. Leukaemia is where the bone marrow has become involved in the cancer
Myeloid Lymphoma/
Lymphoblastic lymphoma
Myeloid lymphomas affected the myeloid cells of the blood i.e. neutrophils, basophils and eosinophils.
Leukocytic lymphomas affect white blood cells[ leukocytes] primarily.
FLT3 inhibitors
Fins-like tyrosine kinase 3[ FLT3] is mutated in 30% of cases and usually carries a very poor prognosis in acute myeloid leukemia.
First generation agents includes primarily Midostaurin which is a non-specific reversible FLT3 inhibitor and will lead to increased side effects. 26,38
Generation 2 agents were developed with the intent to reduce the off-target effects and development of Giltertinib occured. It should be noted that an issue with is that it, has a reduced side effect profile to Midostaurin BUT has no way of targetting other kinases in the scenario that mutations of other kinase pathways were to occur. 38
FLT3-ITD[ a mutant form] will constitutively activate the downward pathways of STAT5, RAS and PI3-K pathways leading to excessive cell proliferation and reduced cell death[ less activity of pro-death elements like NOXA,BAX, Casp9 and P53]. 38
PI3-K Inhibitors
This newly developed agent, Idelalisib, is to be used as second line therapy for the treatent of CLL[ Chronic lymphocytic leukemia alonsgide Rituximab[ binds with CD20 on cancer cells]. This drug specifically binds with PI3-K delta isoform which overcomes one of the issues associated with the pan-PI3-K inhibitors of intolerable side effects. 26,39 Most common form of leukemia in the Western World. 39
PI3-K initates cell growth and proliferation in its activated form
BCR-ABL Tyrosine
Kinase Inhibitors
BCR-ABL fusion protein caused by translocation of Chromosome 9[ ABL gene] onto chromosome 22's BCR gene is present on Chronic myeloid Leukemia cells[ good prognosis] AND acute Lymphoblastic Leukemia[ Poor prognosis]. 40
Imatinib[ Gleevac] is a first in class drug used in the BCR-ABL associated CML and ALL. 26
Generation 2 agents were developed to be effective against mutations that occured against Imatinib and became first line therapy. These agents didn't treat against a common mutation T315I. 40
Generation 3 was developed to treat this in the form of Ponatinib. These agents, however since Gen 1, have an increased risk of causing the patient Thrombosis which may require increased cardiac screening when on these agents. 40
Mantel Cell Lymphoma
Mantel cell lymphoma is an aggressive form of B cell Non-Hodgekins lymphoma[ which is denoted by the lack of a cell type called a Reed Sternberg cell] which is a unique giant cell that lacks many B cell characteristics including the Immunoglobulin gene. 36
Affiliated with CDK hyperactivity. 36
Bruton Tyrosine
Kinase inhibitors
The drug shown is Ibrutinib, involved in the irreversible inhibition of Bruton Tyrosine kinase which prevents the maturation of Pro-B cells to Pre-B cells, thereby preventing multiplication of the and growth of the mutated mantel cells. 37 Second generation irreversible BTK inhibitors have been developed in the form of Acalabrutinib, Zanubrutinib and Tirabrutinib in order to have reduced off-target effects. 37 Ibutinib interacts relatively strongly with EGFR, ITK[IL-2 inducible tyrosine kinase],BMX etc. 37
Non-Small Cell
Lung Cancer
The referenced paper mentions the methods that Tyrosine kinase inhibitors have of treating NSCLC. The mutation of the NPM-ALK genes was one of the first mutations discovered in the causing of cancer and is present in NSCLC in abundance. 19,28
It can be inhibited through the use of drugs like Crizotinib or ceritinib although Crizotinib would be first line use. 28 Interestingly, Anaplastic lymphoma kinase[ ALK] positive cancers of the lungs occur in younger patients who were light smokers or who didnt smoke. 29
EGFR[ Epidermal growth factor receptor] mutation is the second most abundant mutation occuring in NSCLC cells barring KRAF mutation[ KRAF is very difficult to target so it usually is avoided]. Drugs like Erlotinib, Gefitinib may be used in the treatment as EGFR proliferation leads to increased cell proliferation and survivability. 28
Entrectinib may be used as it inhibits tropomyosin receptor kinase[TRK] A and B which contributes toward greater cell survivability. 30
Dabrafenib may also be used as a BRAF inhibitor to treat NSCLC.
Most common form of cancer in non/former smokers and affects mucous producing cells
TRK receptor
Inhibitors
This drug is entrectinib the newest drug to be licensed for the treatment of NSCLC by blocking TRK A and B along with other kinases including ROS1 which is why it is being recommended for use in ROS1 positive NSCLC like Crizotinib but it is 40 times more potent at ROS1 than Crizotinib. ROS1 is a receptor Tyrosine kinase whose mutation is seen in about 3-7% of NSCLC. 30
Larotrectinib also targets the same site but was first in class TRK inhibitor but has less ROS1 activity and is only approved to treat NRTK positive solid tumours. 26
EGFR inhibitors
This drug is Erlotinib, a first generation reversible tyrosine kinase inhibitor against EGFR [ HER1] and this has been iterated upon with the main intention to be active against mutant forms of HER1. The second generation drugs simply aimed to be more effective at EGFR targeting than the first generation with Afatinib and dacomitinib. 28 These drugs did not, however, function well when faced with certain mutations of EGFR like the T790M mutation as mentioned on the video provided. This is where the gatekeeper amino acid of threonine mutates to methionine reducing activity of these drugs due to increased steric hindrance etc. These second gen can still treat exon 19 mutations amongst many others though. 28
This was remedied with Third Generation EGFR inhibitors in Osimertinib which treat patients with T790M mutation and is an irreversible inhibitor due to its acrylamide group. 19,28
BRAF Inhibitor
Dabrafenib may be used in the treatment of NSCLC where BRAF mutation pre-exists. The video provided gives an insight into its utilisation in second line therapy alongside taxanes in cancer therapy
ALK inhibitor
That drug is Crizotinib the first generational ALK inhibitor. This would be iterated upon with the development of Ceritinib which was a second generational ALK inhibitor and following that, Lorlatinib as a third generational ALK inhibitor as it is active against most known ALK mutations. 26,29 With each generational change, increased activity against mutant ALK was found.
Breast Cancer
This may be targeted in a multitude of ways using oral Tyrosine kinase of HER2[ Human epidermal receptor] inhibitors like Lapatinib, Neratinib, pyrotinib, monoclonal antibodies blocking the actual receptor like Trastuzumab and Pertuzumab whose blocking of the HER2 receptor may block Tyrosine kinase activity and it may be targetted. 28
It may be treated by Cyclin Dependent Kinase[CDK] activity from drugs like Abemaciclib and Palbociclib which may be used with aromatase inhibitors to treat HER negative/ER positive breast cancer but further study is being done to assess if Palbociclib treats HER positive breast cancer as a combination therapy.They mainly target 4/6. 28
With regards to CDK[ a serine/threonine kinase] inhibitors, we only use third generation CDK inhibitors[ like Palbociclib shown] which block CDK 4/6 stopping G1/S phase of the cell cycle and have a better side effect profile than the other generations. 28
Generation 1 drugs[Flavopiridol,Roscovitine] could be described as Pan-CDK inhibitors. They showed little effect in cancer and had significant side effects. It should be noted that R-Roscovitine was also being studied to see if it may be used in Cystic Fibrosis but failed in phase 2. 35
Generation 2 bound with CDK 1,2, 5 and 9[ Dinaciclib] selectively but was still found to have a relatively low efficacy with significant side effects. 35
The paper illustrates the SAR for CDK inhibition as well as the affinities for each drug for various CDK's. 35
Oral HER-2
Antagonists
Shown is lapatinib, the first generation of HER2 kinase oral inhibitor. It may be used in combination with monoclonal antibody targeting the HER2 receptor such as Trastuzumab to double the effect against HER2 and may allow for greater coverage against HER2 mutations proven in the NEOALTTO trial. 33
This has been iterated upon in the form of second generation HER2 inhibitors like Neratinib which has an increased affinity for HER2 over Lapatinib so uses a reduced dose and incurs a reduced risk of side effects whilst also being an irreversible inhibitor of HER2. 32 Pyrotinib is also a drug that has been approved in China which has similiar properties to Lapatinib. 34
HER-2 inhibiting
Monoclonal antibodies
Trastuzumab blocks domain 4 of the HER2 receptor whilst Pertuzumab blocks domain 2 of the HER2 receptor preventing interaction and dimerisation of the HER2 receptors. This is less the case with Trastuzumab and the extent of its activity against HER2 is debated as illustrated in the hyperlink. Trastuzumab may be used in combination with maytansine[modified to emtansine] in the form of a antibody drug conjugate illustrated in the picture connected by a MCC linker. 31
The paper provided lists the supposed activity of the trastuzumab and how it may be liable to resistance. 32
Post-translational modifications and how they develop into cancers
As mentioned in the mode of action section, Protein Kinases exhibit their effects by phosphorylation of specific amino acid residues proteins, activating a cascade of intracellular effects. In this section I will discuss two pathways which are activated by post-translational modification of proteins by protein kinases, and whose dysregulation is implicated in some cancers. 22,24
PI3-K
Phosphoinsoitide 3-kinases (PI3Ks) are a family of enzymes which are phosphorylted by protein kinases or GPCR. They mark the beginning of the PI3K/AKT/mTOR pathway, which has many intracellular functions, but of note are cell growth and proliferation. This pathway is commonly implicated in cancers (especially of the breast) and these can arise from mutations of the PI3K (e.g. PIK3CA) or due to upstream activators such as EGFR, which can cause constitutive activation of this pathway, causing unregulated cell proliferation.
B-raf
B-raf is an intracellular protein, which is a member of the Raf kinase family, playing a role in regulating the MAP kinase/ERK signalling pathway. This pathway is important in regulating cell division and differentiation, among other functions. There are possible mutations in B-Raf (e.g. V600E) that can cause dysregulated activation of this pathway, driving unchecked cell proliferation. This mutation is seen in some melanomas.
Drug development
reference 23 -Ciarán
Challenges in drug development
Specificity
Potency to compete with ATP
ATP is present in most cells in millimolar concentrations. Therefore it is difficult to make inhibitors which have the potency to compete with ATP.
ATP-binding pockets
The ATP binding site, within the hinge region of the active site of protein kinases, is largely uniform across the kinase genome. Therefore, some of the unwanted effects of protein kinase inhibitors come from inhibition of kinases that are not in the target tissue. Therefore, many future drug candidates are seeking to develop drug candidates which target specific protein kinases, which is a difficult task.
Dynamic nature of kinome
Overexpression
Overexpression of protein kinases can be associated with unchecked cell proliferation and neoplastic transformation. This is seen in many cases, such as overexpression of cAMP-dependent protein kinase type 1, which is commonly found in those with colon carcinoma cells.
Mutations
Malignant cells can develop mutations in, or around the ATP binding region of the kinase active site, allowing resistance to first line kinase agents. This can commonly be seen by mutation of the gatekeeper residue (e.g. mutation T790M) which sterically inhibits first line agents from binding to the hinge region, while not affecting the binding of ATP. This specific mutation can be seen in EGFR in some non-small cell lung cancers, and may require a third generation protein kinase inhibitor such as Osimertinib. Osimertinib is seen to be effective as the lipophilic substituent that usually binds allosterically near the gatekeeper residue is more flexible than that of an agent like Imatinib and can therefore fit into the binding site regardless of the T790M mutation
Mutation T790M is a common kinase mutation
Resistance
Drug candidates
Rigosertib
Rigosertib is a novel protein kinase inhibitor that doesn't compete for binding in the hinge region of the kinase active site. Instead this agent binds allosterically, which presents a promising target for future drug candidates that look to target mutated kinases. 20,21
Non-ATP competitive inhibitor
Fasudil
Fasudil, a Rho-kinase inhibitor, is being investigated for use as an anti-cancer agent via its inhibition of endothelial cell motility and angiogenesis. 25
Targetting Protein Kinases Therapeutically against Cancer
Protein Kinases are the 2nd most targeted group of drug targets, with GPCRs being the 1st. 11 As most protein kinases promote cell proliferation and migration, when they are constitutively expressed they are associated with oncogenesis. 12
Serine/Threonine Kinase Inhibitors
CDK inhibitors
Cyclin-dependent kinases promote the transition of the cell through the cell cycle. As they have a clear role in driving proliferation, this makes them a target for anti-cancer drug development. However, this has proven to be a challenge, with many CDK inhibitors failing to progress through clinical trials. 10 An example of a successful CDK inhibitor is Pablociclib (Ibrance).
(Ibrance SPC: https://www.medicines.org.uk/emc/product/4449/smpc#gref)
RHO-Kinase Inhibitors
RHO-kinase inhibitors have the potential to stop cancer from spreading by inhibiting cell migration, therefore preventing cancer cells migrating to nearby tissue1
mTOR inhibitors
Mammalian Target of Rapamycin (mTOR) is a protein kinase that regulates several cell processes, including cell growth and proliferation. Dysregulation of mTOR is associated with various cancers, usually caused by a mutation in the PTEN gene. mTOR inhibitors including temsirolimus and everolimus are therefore used to treat some cancers.1
Tyrosine Kinase Inhibitors
More than 70% of the known oncogenes and proto-oncogenes involved in cancer code for protein tyrosine kinases. 6 Receptor tyrosine kinases that can become constitutively activated and therefore oncogenic include EDFR, PDGFR, and VEGFR. Cytoplasmic tyrosine kinases that can become oncogenic include the Src-family and Bruton's tyrosine kinase (BTK) family. 6
Monoclonal Antibodies
Bevacizumab
Bevacizumab is indicated for the treatment of various cancers, including metastatic carcinoma of the colon or rectum. Bevacizumab binds to VEGF, preventing it from binding to its VEGF receptors. This inhibits the formation of new tumour vasculature and inhibits tumour growth. 17
Trastuzumab
Trastuzumab is indicated for the treatment of HER2 positive breast cancer. It is a humanised IgG1 monoclonal antibody which binds to subdomain IV, a juxta-membrane region of the extracellular domain of HER2. It also mediates antibody-dependent cell-mediated cytotoxicity (ADCC). 15
Trastuzumab emtansine
Kadcyla is an antibody-drug conjugate (ADC) that is targeted to HER2. It consists of trastuzumab (humanised anti-HER2 IgG1) which is covalently bonded to the maytansine derivative DM1, which is a microtubule inhibitor, therefore Kadcyla exerts its mechanism of action via both components. As trastuzumab is selective for tumour cells over-expressing HER2, this allows targeted delivery of DM1 to these cells. MCC is a stable thioether linker used to connect the antibody to DM1, and it is this DM1-MCC complex that is referred to as “emtansine”. MCC increases the targeted delivery of DM1 and limits its systemic release. Like trastuzumab, Kadcyla binds to domain IV of the HER2 extracellular domain (ECD) and mediates antibody-dependent cell-mediated cytotoxicity via inhibition of the shedding of HER2 ECD and signalling through the phosphatidylinositol 3-kinase (PI3-K) pathway. Cytotoxic DM1 binds to tubulin of malignant cells and induces apoptosis, along with the trastuzumab component, through cell cycle arrest in the G2/M phase. 16
Small molecules
Small molecule tyrosine kinase inhibitors are usually orally active and exert their action by interfering with the ATP binding site of the catalytic domain of the tyrosine kinase which is oncogenic. 6, 11 They can be classed according to their interaction with the tyrosine kinase, as seen in the table linked.
Reversible
Sunitinib
Sunitinib is indicated for the treatment of gastrointestinal stromal tumour (GIST), metastatic renal cell carcinoma (MRCC), and pancreatic neuroendocrine tumours (pNET) via its action of inhibiting various receptor tyrosine kinases. 18 Its structure consists of an indolinone component (binds to allosteric hydrophobic region of kinase)and a pyrrole ring (binds to hinge region). 6
Imatinib
Imatinib (Glivec) is a cellular tyrosine kinase inhibitor that targets Bcr-Abl tyrosine kinase. 6 It is indicated for the treatment of various cancers, including CML.
Irreversible
Bind covalently with a nucleophilic reactive cysteine residue near the ATP binding site, preventing the entry of ATP and therefore irreversibly inhibiting it. 6, 14 Irreversible tyrosine kinase inhibitors achieve covalent bonding due to the presence of an enone group which can tautomerise. 19
Ibrutinib
Ibrutinib is an irreversible BTK inhibitor, by forming a covalent bond with the cysteine residue in the BTK active site. 6 It is indicated for use in mantle cell lymphoma and chronic lymphocytic leukaemia.
Osimertinib
Osimertinib is a 3rd generation EGFR tyrosine kinase inhibitor and is indicated for use in NSCLC caused by a T790M mutation. 19 It contains the characteristic enone component in an acrylamide group, which allows for irreverisble inhibition. 19
Relevant Modules
Year 5
PF6420
Year 3
PF3013
Year 2
BC2443
PT2448
Year 1
PT1445
Mode of Action
Protein Kinases are enzymes which are responsible for phosphorylation, through the transfer of a terminal phosphate from a high energy donor molecule (typically ATP) to a hydroxyl group on a protein 1.
Signal amplification
Signalling cascades occurs by kinases being activated by regulatory phosphorylation, with one kinase activating the next kinase. Therefore from the activation of one kinase, many downstream kinases all phosphorylate the target protein resulting in signal amplification. 1
Angiogenesis
Anti-apoptosis signalling
Diffrentiation
Proliferation
Amino acid residues
Protein kinases phosphorylate the hydroxyl group on amino acid residues serine, threonine and tyrosine, by recognising a specific amino acid sequence near the residue 1. This results in a conformational change that can affect protein function. 2
Protein kinases can be grouped into 3 classes, based on their characteristic substrate specificity:
- Serine/Threonine protein kinases
- Tyrosine Protein Kinases
- Dual Specificity Protein Kinases (e.g. MAP kinase kinases)
Approximately 86.4% of serine residues are phosphorylated, 11.8% of threonine residues are phosphorylated, while only 1.8% of tyrosine residues are phosphorylated.3
Threonine
Serine
Cytoplasmic Serine/Threonine Kinases
Example includes JNK/MAPK signalling pathway which is activated by extracellular stimuli resulting in cell apoptosis through JUN transcription factor. 8
Receptor Serine/Threonine Kinases
Transduces signals for the TGF-beta family, important in embryo development. 9
Tyrosine
Cytoplasmic Tyrosine Kinases
These are intracellular cytoplasmic proteins that can be membrane-bound or nuclear, which act by relaying intracellular signals. An example is FAK (Focal Adhesion Kinase) which can regulate cell adhesion and proliferation. 8 Mutations in these kinases result in various oncogenic conditions, eg. PI3K mutations are implicated in 30-50% of human cancers. 13
Receptor Tyrosine Kinases
These are enzyme-linked receptors which consist of:1,6
- an intracellular tyrosine kinase domain (containing the catalytic domain)
- transmembrane domain
- extra-cellular ligand binding domain
The ligands for receptor tyrosine kinases are usually proteins including epidermal growth factor (EGF) and platelet-derived growth factor (PDGF).
They are located in the plasma membrane, and upon ligand binding they oligomerise and tyrosine trans-phosphorylation (auto-phosphorylation) occurs in the kinase activation loop. There is phosphorylation of additional sites in the intracellular domain, leading to the docking of a protein substrate and subsequent intracellular signalling cascades. 7
Catalytic Domain
A number of residues within the catalytic domain where the ATP binding pocket is located are highly conserved between the different types of protein kinases, however specific motifs distinguish serine/threonine kinases from tyrosine kinases. 4 In the N-terminal of the catalytic domain, there is a stretch of glycine residues near a lysine residue, which are involved in binding to ATP. There is a conserved aspartic acid residue in the centre of the catalytic domain which is key for the activity of the kinase as an enzyme. 4,5