Mmbrane Proteins
Active Transport- moving substances from an area of low to high concentration, they are doing against the concentration gradient, also uses energy
Cotransport- occurs when active transport of a solute indirectly drives transport of other substances
Sodium-Potassium Pump- this is an example of active transport, where Na+ and K+ Ions of pumped in and out of the membrane through a pump. Typically outside the cell there are more sodium ions and inside there are more potassium. This type of uneven charge distribution creates a voltage difference across membranes.
Resting Membrane Potential/ Equilibrium potential- when neurons are not transmitting signals( same amount of ions going in are coming out)
Electrogenic Pumps- proteins or pumps in membranes that help create a voltage difference across membranes
H+ Pump- transport of protons against their concentration gradient
Unit 2
Celll Signaling
Long Distance Signaling-if the cell releasing the signal is far away from the cell that has the receptor to receive the signal (hormonal signaling)
Receptor-cell that receives the signal molecule does so if it has the receptor
(Membrane receptor
Intracellular Receptor) in the cytoplasm, nucleus a
signal nonpolar molecules can go to receptors Inside the cell, while signal polar can only be received by receptors in the plasma membrane
Local Signaling- form of signaling through physical contact or surface contact where cells can communicate with each other and response (nearby but not touching each other, and communicate by the release of molecules and received by neighboring cells)
Signal Molecule- molecule released by a cell which is recieved by another cell
Intra Cellular moecule- nonpolar and can diffuse through the plasma membrane, therefore molecule binds to the receptor in the cell
Membrane Molecule- Polar molecule /large molecule that can't pass the plasma membrane
Reception, Transduction, Response- Reception is when the molecule binds to the receptor. Transduction is the process of transferring signals throughout an organism. Response is the action that occurs due to the activation of kinase and ATP, and other molecules making a cellular response
Ion Channel Receptor- Ion channels are closed, then a signal molecule binds to the receptor causing the channels to change shape and open. Ions start to flow through until the molecule unbinds, and the channels close
GPCR Signaling- First a polar signaling molecule binds to the inactive GPCR and activates it. Then the G-protein activates which leads to the binding of G protein to the GPCR making the G protein change its shape and kick GDP off and becomes GTP. GTP then slides over to Adenylyl Cyclase and binds to it activating it and it alters it shape, while also becoming GDP again by kicking off a phosphate group with Phosphatases. Then Adenylyl Cyclase turns ATP into cAMP, which then cAMP activate a protein kinase, creating a cellular response.
.Phosphorylation cascade is an example of signal transduction pathway that uses Kinases and Phosphatases.
Adenylyl Cyclases- Enzyme that is used to activate the second messenger cAMP
Protein Kinases: (Adds phosphate groups)Enzymes that catalyze the transfer of phosphate groups from ATP to proteins
Phosphatases-( Removes phosphate groups)an enzyme that removes a phosphate group from proteins
Second messengers- are small, nonprotein, water-soluble molecules or ions that are used in signal transduction to relay a signal within a cell (They are synthesized or released by specific enzymatic reactions, usually as a result of an external signal that was received by a transmembrane receptor and pre-processed by other membrane-associated proteins)
Steroid Hormone Siglaning- the signal molecule is nonpolar so it can diffuse through the plasma membrane, and binds to the receptor inside. Then the receptor goes into the nucleus and taps DNA, which then DNA does transcription of mRNA. The mRNA then leaves the nucleus and is translated into a specific protein
Proteins
Amino acids (monomers)
Main chain
central carbon
R-groups (Side chains)
Acidic amino acids
Negatively Charged
Basic amino acids
Positively charged
Nonpolar amino acids
Carboxyl group (+)
Amino Group(-)
result from the attraction between oppositely charged ions.
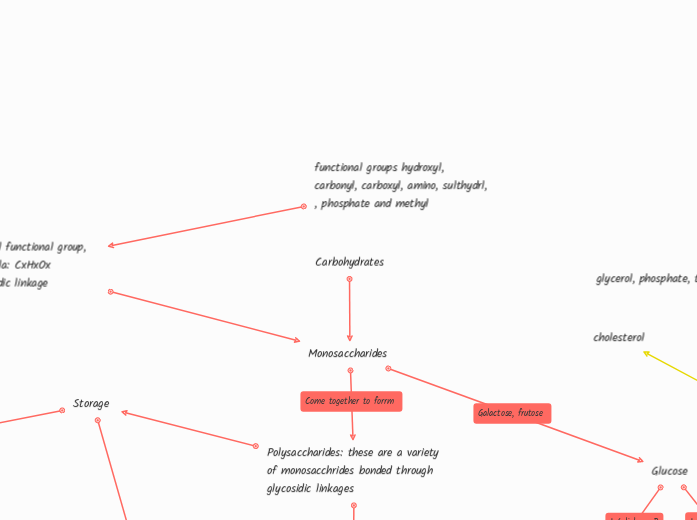
Carbohydrates
Purines: Guaine (G), Adenine(A)
Nucleic Acids
RNA
Ribose sugar
DNA
Deoxyribose sugar
Polymers made of monomers called nucleotides
Nucleoside: Nitrogenous base and pentose sugar
Phosphate group
Phosphodiester bond: The bond in which connects the polymer
Nitrogenous bases
Pyrimidines: Cyosine(C), thymine(T), uracil
Nucleotide: pentose sugar, nitrogenous base, 1 or 2 phosphate groups
glycerol, phosphate, two fatty acids
amphithatic molecule
cholesterol
phospholipid
Lipid
phospholipid
esther bond
double bond
single bond
condensation reaction
A peptide is formed from condensation.
Protein folding
Protein structure: It is determined by amino acid sequence, physical, and chemical conditions in the proteins environment.
Heating a protein up will denature (unfold) the protein to a straight line.
Primary structure- The main chain or amino acids are interacting with peptide bonds. A straight line.
Secondary structure- Folding is beginning to happen it can either be alpha helix or beta pleated sheet. The folding depends on the sequence of the amino acids. Both of these are due to the hydrogen bonds.
Tertiary groups- The folding continues and is folding the secondary structures. Interactions begin between the r groups. The r groups can vary and depending on the r groups depends on the folding of the protein.
Quaternary structure- There are more than 2 polypeptides folded together. Interactions are by the R groups
If there are hydrophobic interactions the protein would fold inwards letting the hydrophobic interact on the inside with other hydrophobic r groups. The hydrophilic interactions would be outwards interacting with other hydrophilic r groups.
The R-group interactions are hydrophobic, hydrophillic, disulfide, van der waals, ionic, and hydrogen bonds.
Hydrophobic interaction
enantiomer
geometric
structural
isomers
trans bond
cis bond
unsaturated fat molecule
saturate fat molecule
triaglycerol
glycerol molecule+ fatty acid
Unit 1
Chemical bonds: An attraction between two or more atoms, and is what forms a chemical
Molecules: A group of atoms connected by bonds
Polar molecules: Is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively charged end.
Polar amino acids
Nonpolar molecules: When atoms bond together to form molecules, they share or give electrons. If the electrons are shared equally by the atoms, then there is no resulting charge,
Van der Waals
Ionic Bonds
covalent bonds
bonds are being equally shared between atoms
Hydrogen bonds
functional groups hydroxyl, carbonyl, carboxyl, amino, sulthydrl, , phosphate and methyl
Carboxl, hydroxl functional group, chemical formula: CxHxOx Link by glycosidic linkage
Monosaccharides
Glucose
Beta Glucose
Same chemical formula, different structure arrangement of OH
Cannot be broken down by enzymes
Alpha Glucose
Can be broken down by enzymes
Polysaccharides: these are a variety of monosacchrides bonded through glycosidic linkages
Storage
Glycogen: animals
1-6 Glycosidic linkage
Alpha Glucose, 1-4 glycosidic linkage, branching, easy to break
Starch: plants
Amylopectin
Branching, Alpha glucose, 1-4 glycosidic linkage, can be easily broken
Amylose
No branching, Alpha glucose, 1-4 glycosidic linkage, cannot be easily broken
Helical shaped
Structure
Cellulose
Beta glucose, 1-4 beta glycosidic linkage, no branching, Plant cell