surfactants
liposome
aqueous core surrounded by
one or more bilayer membranes
alternating with
aqueous compartment
form at an appropriate
lipid- to - water
ratio and temp
formed by phospholipids
via: signification
homogenization
ultrasonification
NOT spontaneous
two hydrophobic chains that
tends to form lamellar phase
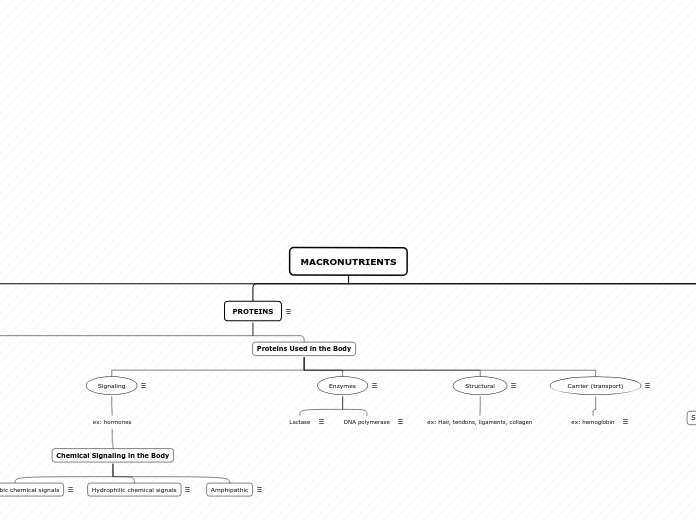
drug delivery for
both hydrophobic
and hydrophilic
good for antibodies and vaccination
issue
tissue distribution
vesicle clearance rate
low stability in blood
and interstitial fluids
classified based on their
size and the number of lamellae
MLV
multomellar vesicles
LUV
Large unilamellar vesicles
SUV
small unilamellar vesicles
Classification:
HLB
*** memorize the right side
HLB
Antifoaming agent 2-3
w/o emulsifying agents 3-6
wetting and spreading agents 7-9
o/w emulsifying agents 8-16
detergents 13-15
solubilizing agent 15-18
HLBmix= Fa(HLBa)+Fb(HLBb)
low HLB= more lipophilic
higher HLB= more hydrophilic
range 0-20
**emulsifier must be more
soluble in continuous phase
surfactant as emulsifying agent 8-18
7-9 surfactant act as wetting agent
3-6 W/O
8-18 O/W
hydrophilic-lipophilic balance
by its hydrophilic group
( polar end )
non ionic
poor as a surfactant
may be water soluble or insoluble
depending on functional group
water insoluble
fatty acid
glycerol esters
fatty alcohols
water soluble
polyoxyethylene groups
stability
good compatibitlity
no charge
Zwitterionic
isoelectric point
behave similar to nonionic surfactant
both positive and negative
charges upon full ionization
carboxylate
ammonium group
example
alkyl betaine
glycine
Cationic
Benzylalkonium chloride
( BAC/BAK)
function in protonated state
positive charge
quaternary ammonium
retains charge
over the entire pH range
amines
ph depended
nitrogen atom
Anionic
above the pKa will ionize
if countering are mono valiant most likely not soluble
if the counter ion are sodium or potassium= water soluble
in a basic solution acid are negatively charge
Sodium Lauryl sulfate (SLS)
most common surfactant
emulsifying agent
wetting agent
most widely used class
negative charge
phosphate
sulfonate
sulfate
caboxylate
clinical applications
lung surfactant
premature infant have not enough lung surfactant= death
decreasing the surface tension
decreases pressure inside alveoli
prevents collapse of alveoli
covers the surface of alveoli contacted with air
nonionic often aid in bacterial growth
antibacterial
cationic and ionic absorb onto bacteria surface
--> leaking of lipid cell membrane--> bacterial death
liposomes
antibodies
IM/ Subq: liposome get into the lymphatic system;
vaccine delivery
reaction with antibodies to be
phagocytize into the liver and
spleen allowing for passive targeting
of these sites
*surface- active agents?
surfactant
surfactant reduces surface tension
surface tension
surface activity
this is to achieve minimum energy state
amphiphilic orientation to remove the
hydrophobic group from the aq. environment
lowering the surface tension
at the surface particle are not completely surrounded
which results in a net inward force attraction exerted on
molecules at the surface by the molecules in the bulk solution
contracted surface = minimum free energy
thus liquid surface contracts and
form a sphere spontaneously
lower the interfacial tension between
the phases (oil and water) blending
enable interaction of two phase
miceller formation
wetting
micelle
shape
nonionic surfactants = lower cmc= larger micelle
mostly affected by hydrophobic
region and any steric interactions
increase in hydrophobic chain
bigger size
low CMC
depend upon surfactant's molecular geometry
drug delivery for hydrophobic materials
hydrophilic shells
hydrophobic core
spontaneous
Surface tension decreases until CMC
critical micelle concentration
More surfactant= less surface tension
surfactant associating to itself
encapsulating and forming a sphere
hydrophobic groups withdrawing
form the aqueous phase
concentration that micelles form
to attain minimum free energy state
too much surfactant requires energy
to keep all in a solution
thus to lower energy form micelle
= Entropic Effect
definition
emulsion formation
Wetting
contact angle
high = poor wettability > 90
low = good wettability <60
the extent to which a solid
will physically interact with a liquid
lowers the interfacial tension
between phases enables interaction
surface active agents amphiphilic in nature
hydrophobic head
hydrophilic region