a Amellia Chartrand 3 éve
512
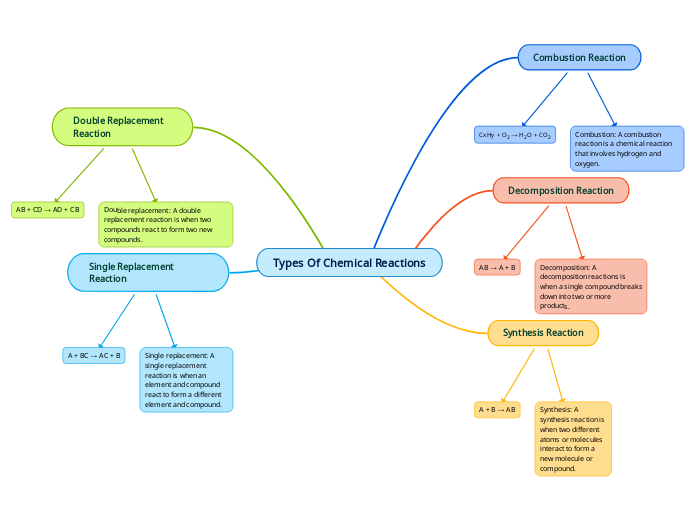
Types Of Chemical Reactions
Chemical reactions can be categorized based on how reactants transform into products. In combustion reactions, hydrogen and oxygen interact to produce water and carbon dioxide. Synthesis reactions involve the combination of two different atoms or molecules to form a new compound.